- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Robotic Surgery For Prostate Removal Could Be The Solution To Erectile Dysfunction
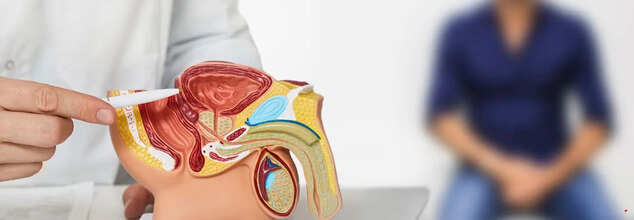
Prostate Cancer (Credit: Canva)
Prostate cancer is one of the most common cancers in the US, besides skin cancer. Worldwide, it is the 4th most common form of cancer. While it is treatable, it leaves a risk of erectile dysfunction.
However, surgeons in Texas, US performed a groundbreaking surgical milestone when they successfully performed the world's first dual-robotic surgery to remove a prostate gland. The dual-robotic method reduces the number of incisions, leading to less pain, faster recovery, fewer complications, and minimal scarring. This groundbreaking approach, aimed at preserving nerves essential for erectile and urinary functions, has ushered in a new era of hope for medical science.
"We’ve incorporated magnetic technology that enhances tissue retraction and visualization," said Dr. Alberto Rodriguez-Navarro, founder and CEO of Levita Magnetics (the surgical system used). "For prostate surgeries, this allows the surgeon to see and preserve nerve bundles critical for preventing urinary incontinence and maintaining sexual function," he added.
Surgery Was Performed On A 67-Year-old
Dr Jeffrey Cadeddu, a urologist at UT Southwestern Medical Center, utilized the da Vinci single port robotic system and Levita’s MARS platform technology to perform a robotic prostatectomy on a 67-year-old man with Stage 2 prostate cancer. The procedure involved making a single small incision in the abdomen to insert surgical tools and a camera.
"The da Vinci system provides deep access to tissue with centralized instruments, while the MARS platform uses magnetic forces for precise tissue manipulation," Dr Cadeddu told a media organization. "In this procedure, one robot managed tissue retraction, and the other controlled cutting tools, creating a seamless integration of technologies.”
This approach marks a shift in robotic surgeries, with technologies from different manufacturers working in tandem for enhanced precision. The MARS system, launched in 2023, has already been used for weight-loss surgeries, gallbladder removals, and colorectal procedures.
ALSO READ: These US States Were Hit Hardest By COVID
What Does Prostate Cancer Mean?
Prostate cancer is a type of cancer that occurs when malignant cells form in the prostate gland, which is a walnut-sized gland in the male reproductive system. Prostate cancer treatment guidelines have shifted their path a bit in recent years, with many men opting for active surveillance rather than immediate treatment for slow-growing tumours. However, about 50% of men on "watchful waiting" will require further treatment within 5 years because of the tumour progression. This is what triggered many researchers to aim and identify whether dietary modifications, specifically increasing omega-3 fatty acids, could prolong this surveillance period and slow down the tumour progression.
Prostate cancer that's more advanced may cause signs and symptoms such as:
- Trouble urinating
- Decreased force in the stream of urine
- Blood in the urine
- Blood in the semen
- Bone pain
- Losing weight without trying
- Erectile dysfunction
Over 40% Prostate Cancer Patients in India Diagnosed After Cancer Has Spread: ICMR study

Credit: Canva
A recent study by the Indian Council of Medical Research (ICMR) showed that more than 40 percent of prostate cancer patients in the country are diagnosed after the cancer has spread.
The 43 percent of late diagnosis cases indicates the significant burden of late detection of prostate cancer in the country. This can not only limit treatment options but also lead to poorer survival outcomes.
The study, published in the Indian Journal of Surgical Oncology, revealed that while more than 80 per cent began treatment within two months, but referral patients experienced longer delays.
Researchers from the ICMR’s National Centre for Disease Informatics and Research, in Bengaluru, stressed the need to strengthen referral pathways to ensure timely, stage-appropriate care.
“Our study indicates that over 80 percent of patients commence treatment within two months of diagnosis, but referral systems and delays in care persist,” said corresponding author Prashant Mathur, Director, ICMR-NCDIR, in the paper.
“To address these challenges, the healthcare system must prioritize improving referral efficiency, reducing administrative bottlenecks, enhancing coordination through digital health records, and multidisciplinary tumor boards,” the authors added.
Age, The Strongest Risk Factor For Prostate Cancer
The ICMR study is based on an analysis of 9,347 cases from 96 hospitals under the National Cancer Registry Program.
The researchers found that 75.6 percent of total prostate cancer cases occurred in the age group of 60–80 years, indicating that advanced age remains the biggest risk factor for the condition.
As life expectancy increases, more men reach the higher-risk age group, but awareness and screening practices have not scaled proportionately.
Adenocarcinoma was the most common pathology, constituting 77 percent of cases.
It is the most common form of prostate cancer, accounting for over 95 per cent of all cases. It develops in the gland cells that produce prostate fluid and typically grows slowly over several years.
Further, the ICMR researchers noted that about 57 percent of cases were diagnosed with localized (29.9 percent) or locoregional (27 percent) cancer.
Thirty percent underwent surgical treatment, and 22 percent received radiation therapy. Systemic therapy was the most common single modality treatment.
“Early detection and streamlined referral pathways are essential to improve prostate cancer outcomes in India,” the researchers said.
Prostate Cancer And Its Prevalence In India
Prostate cancer forms in the cells of the prostate -- a gland found only in males and a part of the male reproductive system. It lies below the urinary bladder and in front of the rectum.
Nearly all prostate cancers develop from glandular cells (adenocarcinomas).
Globally, prostate cancer is the most frequently diagnosed cancer among men in 112 countries and the leading cause of cancer death in 48 countries. In 2020, an estimated 1.4 million new cases of prostate cancer and 0.37 million deaths were reported worldwide.
In India, it is the second most common cancer among men, accounting for more than 60 percent of the prostate cancer burden in South-Central Asia. As per the ICMR data, the country reported 34,540 incidences of prostate cancer and 16,783 deaths.
Prostate cancer symptoms include urinary difficulty, a weak stream, or blood in the urine.
As prostate cancer is a slow-developing disease, it often causes no symptoms during the early-stage, leading to delayed medical consultation.
Other reasons for late detection in India include low awareness, limited routine screening -- PSA (prostate-specific antigen) programs; social stigma and hesitation due to embarrassment or cultural taboos.
Lack of access to specialist care, diagnostic facilities, and cancer centers, especially in rural populations, coupled with cost and referral gaps, also leads to delay in diagnosis.
Zepto Customer Claims To Find 'Plastic' Inside Eggs, FSSAI Says Impossible

Credit: Instagram/ @climbwithshalini
In a shocking video, a woman who ordered packaged eggs sold by Eggoz from Zepto claims they were filled with plastic-made strands instead of yolk, however the brand soon quoted FSSAI's rules and issued a clarification.
Earlier this week, Shalini Singh shared an Instagram video of six eggs that appear to have yellow, thread-like strands emerging from within, suggesting that instead of slimy yolk, she found a plastic substance bursting out of the eggs after boiling them.
In the clip, the visibly upset customer is heard saying: “Until now we had only heard that fake eggs or plastic eggs were being sold in the market, but today I witnessed an example in my own home. I ordered Eggoz Everyday brand eggs from Zepto and as soon as I put them on to boil, instead of yolk, plastic started coming out from inside."
Pointing at the cracked eggs, she added, “You can see in the video yourself, plastic is coming out in the form of noodles. Each egg has the Eggoz Everyday stamp on it. Look at how plastic fake eggs are reaching our homes.
“This is what we are buying today. If they had been cooked in another way, we might not even have realized we were consuming plastic. These boiled eggs burst, and that is how we found out it was plastic. If this is the condition of such big brands today, what is even left safe to eat?"
Can Egg Yolks Be Filled With Plastic?
According to the Food Safety and Standards Authority of India (FSSAI), it is impossible for eggs to be made artificially or with plastic. In a guidance titled 'Eqq Quality And Safety', the agency wrote: "Plastic eggs or artificial eggs are a myth mainly due to the fact that there is no technology available to produce a plastic/ artificial egg that would perfectly resemble a natural egg."Consumers need to remember that the quality and appearance of the egg mostly depend on the way they are stored and for how long they are stored. Egg quality is best maintained when they are stored in cold temperatures preferably inside refrigerators and consumed within a period of 2 to 3 days.
"When kept in room temperature, several changes take place in an egg that bring about differences in the smell, texture and appearance of the egg."
The FSSAI further suggested holding up an egg against a very bright light in a dark room to detect its age. If its fresh, the egg will display a small air gap usually at the broader (blunt) end and if its starting to age, the air cell will expand in volume.
Upon hard boiling a fresh egg, you can clearly see the indentation left behind at the top of the egg once the shell is peeled off.
When fresh eggs are boiled, the yolk normally stays in the center and is not very mobile because of the chalaza (the strings of tissue) that hold it in place. These strings break down as the egg ages (during storage or transportation). When hard boiled eggs are cut length wise, one can see that the yolk has moved off the centre.
What Did The Company Say?
In response to the video, Eggoz said: “We’re really sorry to hear about your experience and completely understand your concern. This is not the kind of situation we ever want our customers to face. Kindly DM us your contact information and our customer support team will connect with you to resolve this at the earliest."Thank you for bringing this to our attention."
They also reached out with an official clarification, stating that there is no such thing as plastic eggs and that the claims stem from common misconceptions, quoting FSSAI's guidance.
In an official statement, the brand commented: "There are no plastic eggs. Eggs are a natural agricultural product, and variations in texture or appearance may occur due to storage or temperature conditions at different stages after production. Such natural changes do not indicate the presence of artificial or plastic material and do not affect food safety."
The company also highlighted its commitment to strict farm-to-shelf quality processes, including hygienic handling, batch traceability and temperature-controlled logistics.
Denmark 1st European country to eradicate mother-to-child transmission of HIV, syphilis
Credit: WHO
Denmark has become the first country in the European Union (EU) to eliminate mother-to-child transmission (EMTCT) of HIV and syphilis -- two serious, and often co-occurring, sexually transmitted infections (STIs).
The World Health Organization (WHO) validated Denmark for the EMTCT of HIV and syphilis, for low transmission rates and high coverage of prenatal testing and treatment for pregnant women from 2021 to 2024.
“The elimination of mother-to-child transmission of HIV and syphilis marks a major public health achievement for Denmark,” said Dr Tedros Adhanom Ghebreyesus, WHO Director-General.
“This milestone demonstrates that with strong political commitment and consistent investment in primary care and integrated maternal and child health services, countries can protect every pregnant woman and newborn from these diseases,” he added.
With the recognition, Denmark is now among 22 other countries and territories validated by WHO for the elimination of mother-to-child transmission of HIV, syphilis, or hepatitis B virus.
How Denmark Achieved EMTCT Of HIV And Syphilis
Elimination can be defined as testing and treating at least 95 out of every 100 pregnant women. This also includes keeping new infant infections below 50 per 100,000 births, year after year.
"Denmark has met these benchmarks through strong antenatal care, reliable data systems, and respect for women's rights. We will support Denmark as it works toward full triple elimination, when it adds hepatitis B," said Dr. Hans Henri P. Kluge, WHO Regional Director for Europe.
"This validation by WHO is a proud moment for Denmark and the result of decades of work by our health-care professionals, midwives, and public health teams to ensure that every pregnant woman receives the screening and care she needs,” added Sophie Løhde, Minister for the Interior and Health, Denmark.
Denmark has low rates of HIV and syphilis among pregnant women. While 5,950 people are living in the country with HIV, less than 0.1 percent of pregnant women are affected. With regular testing and treatment, mother-to-child transmission was reduced to zero.
Further, the systematic prenatal screening and care also reduced the cases of congenital syphilis (syphilis passed from mother to baby). In 2024, the country reported 626 cases of syphilis, more in men (524) than in women (102).
The country is now on track towards validating hepatitis B virus elimination.
The prevalence of chronic hepatitis B infection in Denmark is estimated at around 0.2–0.3 percent, mainly among migrants from endemic regions.
In October 2025, the Maldives became the first country in the world to achieve ‘triple elimination’ of mother-to-child transmission of hepatitis B, HIV, and syphilis. The country had achieved WHO validation for EMTCT of HIV and syphilis in 2019.
Global Prevalence Of HIV And syphilis
Globally, more than 39 million people were living with HIV in 2022, and over 20 million cases of syphilis were reported among women of childbearing age by 2021.
Syphilis sores create entry points for HIV, while HIV can accelerate syphilis progression.
While syphilis is curable with antibiotics, HIV is manageable but not curable.
The sexually transmitted infections are also increasing in prevalence worldwide.
More than 1 million curable STIs are acquired every day worldwide in people 15–49 years old, the majority of which are asymptomatic.
STIs have a direct impact on sexual and reproductive health through stigmatization, infertility, cancers, and pregnancy complications, and can increase the risk of HIV.
© 2024 Bennett, Coleman & Company Limited

