- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Tiny 3D-Printed Spinal Cords Could Reverse Paralysis, How Did Scientists Make It Work?
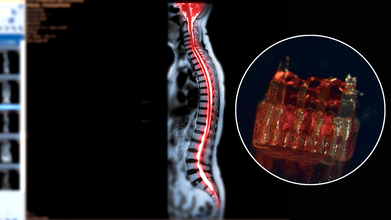
Credits: Canva/McAlpine Research Group, University of Minnesota
Spinal cord injuries have long posed one of the most stubborn challenges in medicine. Affecting more than 300,000 people in the United States alone, these injuries often lead to permanent paralysis because damaged nerve fibers fail to regenerate across the site of trauma. Traditional therapies focus largely on rehabilitation and symptom management rather than reversing the underlying injury. Now, a groundbreaking study from the University of Minnesota Twin Cities suggests that a combination of 3D printing, stem cells, and lab-grown tissues could change that narrative. Researchers have engineered tiny scaffolds that guide stem cells to form nerve fibers capable of bridging severed spinal cords. In rat models, this approach restored nerve connections and movement—offering a tantalizing glimpse into the future of paralysis treatment.
At the heart of this innovation are organoid scaffolds—microscopic 3D-printed structures designed to direct stem cell growth. These scaffolds contain a network of tiny channels that can be seeded with spinal neural progenitor cells (sNPCs). Originating from human adult stem cells, sNPCs have the potential to differentiate into the various types of neurons needed for spinal cord repair. The scaffold essentially provides a framework for these cells, ensuring they grow along the correct pathways to reconnect disrupted nerve circuits.
Guebum Han, a former postdoctoral researcher in mechanical engineering at the University of Minnesota and the study’s first author, explains, “We use the 3D printed channels of the scaffold to direct the growth of the stem cells, which ensures the new nerve fibers grow in the desired way. This method creates a relay system that, when placed in the spinal cord, bypasses the damaged area.”
To test their approach, the researchers transplanted the scaffolds into rats with completely severed spinal cords. Over time, the stem cells differentiated into mature neurons and extended their nerve fibers in both directions—toward the head (rostral) and toward the tail (caudal)—forming new connections with the host’s existing spinal circuitry.
The results were remarkable. Rats that received the organoid scaffolds showed significant functional recovery compared to controls, regaining movements that were previously impossible. The new neurons integrated seamlessly into the host tissue, demonstrating that lab-grown spinal tissue could not only survive transplantation but also restore communication across previously severed areas.
Can Lab-Grown Organs Help Patients?
While the research is still in its early stages, the potential implications for human medicine are profound. Spinal cord injuries have been notoriously resistant to treatment because adult nerve cells rarely regrow once damaged. This study provides proof-of-concept that targeted, scaffold-guided stem cell growth can rebuild the neural network necessary for motor function.
Ann Parr, professor of neurosurgery at the University of Minnesota, emphasizes the significance: “Regenerative medicine has brought about a new era in spinal cord injury research. Our laboratory is excited to explore the future potential of our ‘mini spinal cords’ for clinical translation.” The team hopes to refine the technique, scale up scaffold production, and move toward clinical trials that could one day benefit people living with paralysis.
Despite the promising results, several hurdles remain before this technology can be applied to humans. Scaling the tiny lab-grown spinal cords to the size necessary for human injuries will require sophisticated bioengineering solutions. Immune rejection and integration into a complex, pre-existing nervous system present additional challenges. Moreover, safety and efficacy will need to be rigorously tested in larger animal models before human trials can proceed.
The ethical considerations of stem cell use and genetic manipulation also require careful navigation. While adult stem cells used in this study bypass some of the ethical debates associated with embryonic stem cells, clinical applications must still adhere to stringent regulatory standards.
The merging of 3D printing, stem cell science, and lab-grown tissue engineering represents a paradigm shift in regenerative medicine. The concept of “mini spinal cords” could open the door to therapies not only for spinal cord injuries but potentially for other neurodegenerative diseases that involve nerve degeneration, such as amyotrophic lateral sclerosis (ALS) or multiple sclerosis.
Moreover, these technologies exemplify the broader trend of personalized medicine. By tailoring organoid scaffolds to individual patients, it may become possible to repair nervous system injuries with unprecedented precision. This could drastically improve outcomes, reduce rehabilitation times, and enhance quality of life for patients who currently have few options.
The University of Minnesota study is an early but significant step toward reversing paralysis. By combining 3D-printed scaffolds, stem cell biology, and lab-grown spinal tissue, researchers have demonstrated that damaged neural pathways can be rebuilt and functional recovery is achievable—at least in animal models.
While human applications are still a way off, the research provides a blueprint for the future of spinal cord repair and regenerative neuroscience. For the millions affected by spinal cord injuries, these tiny lab-grown spinal cords could one day offer more than hope—they could offer a pathway to regained movement and independence.
FDA Issues Live It Up Super Greens Supplement Powder Recall Over Salmonella Outbreak

Credit: Canva
Health officials have issued a recall for the Live It Up Super Greens supplement powder after 45 people across 21 states were found suffering from salmonella across the US.
The Food and Drug Administration and Centers for Disease Control and Prevention issued an official recall on January 14 for the Live it Up Original and Wild Berry Super Greens dietary supplement powder flavors.
The affected products with expiration dates from August 2026 and January 2028 have been affected by this recall and include:
- Live it Up Super Greens, NET WT 8.5 oz (240g) with UPC 860013190804.
- Live it Up Super Greens, 30 – 0.28oz (8g) sticks, NET WT. 8.47 oz (240g) with UPC 850077468063
- Live it Up Super Greens, Wild Berry, NET WT 8.5OZ (240g), with UPC 860013190811
- Live it Up Super Greens, Wild Berry, 30 – 0.32oz (9g) Sticks, NET WT. 9.52oz (270g), with UPC 850077468070
Authorities has advised consumers to not eat, sell or serve the affected Live it Up-brand products and to contact the company for returns.
Additionally, officials are asking people to wash items and surfaces that may have touched the recalled super greens supplement powders using hot soapy water or a dishwasher.
Of the 45 illnesses, 12 resulted in hospitalizations, according to the FDA. No deaths linked to the recall have been reported.
What Is Salmonella?
Salmonella, or salmonellosis, is an infection caused by the Salmonella bacteria which can invade and destroy the cells that line the intestines, resulting in low water absorption. This makes it hard for the body to absorb water, which can give you stomach pains, diarrhea and fever.Apart from this, other symptoms include:
- Fever
- Nausea
- Vomiting
- Chills
- Headache
- Blood in the stool
Most people develop symptoms within 8 to 72 hours after exposure while most healthy people recover within a few days to a week without specific treatment. However, in some cases, diarrhea can cause severe dehydration and requires prompt medical attention.
While anyone can contract salmonella, children younger than five, elderly and people with weakened immune systems are more likely to develop severe infections.
Is Salmonella Life-Threatening?
Life-threatening complications may develop if the infection spreads beyond the intestines to other organs. The risk of getting salmonella infection is higher with travel to countries without clean drinking water and proper sewage disposal.
While most people do not require medical attention for salmonella infection, those at high risk may need a health care provider if the infection lasts more than a few days, is associated with high fever or bloody stools and appears to be causing dehydration, with signs such as such as urinating less than usual, dark-colored urine as well as having a dry mouth and tongue.
This Blood Test May Reveal The Right Breast Cancer Treatment Before Therapy Begins

Credits: Canva
Scientists have created a straightforward DNA blood test that can help predict how effectively people with breast cancer will respond to treatment. Each year, more than two million individuals worldwide are diagnosed with the condition, making it the most common cancer globally. While treatment options have advanced over the years, doctors still face challenges in identifying which therapies will work best for each patient.
Researchers have now developed a liquid biopsy that can indicate how likely a patient is to benefit from a particular treatment, even before therapy starts. This development could mark a major shift in care, as it may allow patients to move sooner to better options and avoid treatments that are unlikely to help, improving their chances of recovery. Below, we look closely at the study that explains how this blood test works.
New Blood Test Can Predict Breast Cancer Treatment
People with breast cancer could soon benefit from a new blood test that predicts treatment response before therapy begins, offering hope for better outcomes and improved quality of life. Scientists have designed a liquid biopsy that examines small fragments of cancer DNA circulating in the bloodstream. This allows doctors to see which treatments are most likely to be effective for each patient. Reported in the journal Clinical Cancer, the test could help clinicians steer away from ineffective therapies, shift patients to more suitable options earlier, and personalise treatment decisions much sooner.
How Does Breast Cancer Spread?
Breast cancer develops when cells in the breast begin to grow abnormally and form a dangerous lump. It most often starts in the milk ducts or milk-producing glands and can spread to other areas of the body if it is not treated early. The disease occurs when changes in breast cells cause them to multiply uncontrollably. These changes may be inherited or develop over time. Known risk factors include increasing age, a family history of breast cancer, hormonal factors, excess weight, alcohol consumption and physical inactivity. In some cases, no clear cause can be identified.
According to the World Health Organisation (WHO), breast cancer is the most common cancer worldwide.
What Does The Research Reveal?
The research, led by scientists at the Institute of Cancer Research, examined patients with advanced breast cancer. Blood samples were used to measure levels of circulating tumour DNA, which is genetic material released into the bloodstream by cancer cells, both before treatment began and again after four weeks.
Researchers then analysed how these ctDNA levels related to patient outcomes, including tumour response to treatment and the length of time the cancer remained controlled. The findings showed a clear association between low ctDNA levels and better responses to treatment. Patients who had low or undetectable ctDNA either at the start of therapy or after just one cycle were far more likely to respond positively.
Participants were split into two groups based on cancer type and genetic mutations. The first group included patients with mutations such as ESR1, HER2, AKT1, AKT or PTEN, who were treated with targeted therapies designed to match their specific genetic changes.
The second group involved patients with triple-negative breast cancer, a particularly aggressive form that lacks targetable mutations. These patients were treated with a combination of the PARP inhibitor olaparib and the ATR inhibitor ceralasertib.
Among those with triple-negative breast cancer, patients who had low ctDNA levels before treatment saw longer periods where the disease did not worsen, averaging 10.2 months compared with 4.4 months in patients with higher ctDNA levels.
Treatment response rates were also notably higher. Tumours shrank or disappeared in 40 per cent of patients with low ctDNA, compared with only 9.7 per cent of those with higher levels.
In patients receiving targeted therapies, a similar but less pronounced pattern was seen before treatment began. However, results after four weeks were particularly strong. Patients whose ctDNA levels dropped to undetectable levels experienced far better outcomes, with cancer control lasting 10.6 months compared with 3.5 months in those whose ctDNA remained detectable.
In the triple-negative group, patients whose ctDNA disappeared after four weeks had their disease kept under control for 12 months, compared with 4.3 months for those whose ctDNA stayed detectable. Treatment response in this group rose sharply to 85.7 per cent, versus 11.4 per cent among patients with detectable ctDNA.
Measles Outbreak Confirmed In Two South Carolina College
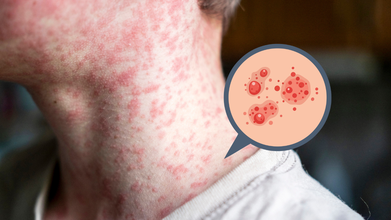
Credits: Canva/iStock
Measles reached two South Carolina colleges, Anderson University and Clemson University. On Monday, Anderson University confirmed that one of the studies was diagnosed with measles and may have exposed others with the same. The student is no longer on campus. Authorities have asked anyone who think are exposed to stay home and not show up in class, work, or in public areas. The students are also asked to call Thrive Wellness Center at 864-622-6978 before visiting in person to avoid spread as measles is highly contagious.
Clemson University whereas, over the weekend, announced the South Carolina Department of Public Health of the case. The person infected is in quarantine.
As per the data from the Student Health Services, nearly 98% of campus students have provided proof of immunity, said the university.
What Is Measles?
Measles, also known as rubeola, is a highly contagious viral illness that typically causes fever, cough, a runny nose, red and watery eyes, and a distinctive red, blotchy rash that usually begins on the face and spreads downward. The virus spreads through the air when an infected person coughs or sneezes and can lead to serious complications such as pneumonia or brain inflammation. Despite its severity, measles is preventable through a safe and effective vaccine, as per the Mayo Clinic.
Read: Kentucky Reports First Positive Measles Case of 2026: Confirmed Health Officials
How Contagious Is Measles?
Measles is among the most contagious diseases in the world. The virus spreads through airborne droplets that can linger in the air or on surfaces for hours. Up to 90% of unvaccinated people who are exposed to measles will become infected. A single infected person can pass the virus to an estimated 12 to 18 others through close contact or shared spaces. People can transmit the virus days before symptoms become obvious and continue spreading it after the rash appears, according to the World Health Organization.
How Long Is Someone Contagious With Measles?
Someone infected with measles can spread the virus from four days before the rash develops to four days after it appears. The virus spreads so efficiently that about 90% of people who are unvaccinated or have never had measles will become infected after being exposed.
In November, Canada lost its measles elimination status following a significant outbreak, according to the Pan American Health Organization, which works closely with the World Health Organization.
“It’s important to say that all the other 34 countries in the region, they keep their certification as measles-free,” said PAHO/WHO Director Dr. Jarbas Barbosa at the time, as per NPR News.
U.S. health officials have also warned that genetic links between outbreaks in different states suggest continued spread.
“The trajectory that we’re looking at now is that we do anticipate more cases well into January,” Bell said. “What that means for us nationally in terms of how they are defining our designation in this country as having eliminated measles is unclear.”
Also Read: Measles Symptoms Explained: Can The Infection Be Deadly?
Measles Symptoms Develop In Three Stages
According to the Mayo Clinic, measles symptoms usually appear in three distinct stages.
Stage 1: Incubation period (10 to 14 days)
During this phase, there are typically no noticeable or warning symptoms.
Stage 2: Early symptoms begin
Symptoms at this stage may include a dry cough, fever, red and inflamed eyes known as conjunctivitis, a runny nose, and a sore throat.
Stage 3: Acute illness and rash
“In the third stage, a rash begins to develop, usually starting on the face. Small white spots called Koplik spots may appear inside the mouth two to three days after symptoms first appear,” the Mayo Clinic explains. “The measles rash typically shows up three to five days after the initial symptoms.
“Over the following days, the rash spreads to the arms, torso, and legs. Alongside the rash, fever often rises rapidly and can exceed 105 degrees Fahrenheit,” the guidance continues. “Eventually, the fever subsides, and the rash fades from the body starting at the head and moving downward.”
Is Measles Deadly?
Yes, measles can be deadly and carries a significant risk of death, according to the Centers for Disease Control and Prevention.
“Measles can lead to serious health complications, including pneumonia, inflammation of the brain known as encephalitis, and death,” the CDC states. “Between one and three out of every 1,000 people infected with measles will die. Around one in five people with measles will require hospital care, and one in every 20 children with measles develops pneumonia, which is the leading cause of measles-related deaths in young children.
“One in every 1,000 people with measles will experience brain swelling, which can result in permanent brain damage.”
© 2024 Bennett, Coleman & Company Limited

