- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Canada Top Court Says COVID Travel Restrictions Broke Mobility Rights Within The 'Reasonable Limits'

Credits: iStock and Canva
Travel Restrictions To Canada: The top court of the land, that is the Supreme Court of Canada ruled that travel restrictions were violative of citizen's right, however, are reasonable during "grave emergency" like pandemic.
Travel Restrictions To Canada: What Happened That Led To Supreme Court Ruling?
During May 2020, in early days of the COVID-19 pandemic, Kimberley Taylor, who was living in Halifax, got the news of her mother Eileen's passing away at the age of 75 of natural causes at St John's, as reported by The Global Mail. Kimberley wanted to attend a small memorial service however was unable to go there due to the Newfoundland government's rejection of her initial request.
The memorial service and the burial was attended by her father, sister and niece. "I was denied the ability to join my family to grieve my mother," she said, as reported.
This is when she along with the Canadian Civil Liberties Association launched a legal challenge. They noted that strict travel restrictions were in violation of the mobility rights within Canada as guaranteed by Section 6 of the Charter of Rights and Freedoms.
Last week, on Friday, a nine judge bench in the Supreme Court of Canada provided an expansive view of the Canadians' Charter mobility rights.
Travel Restrictions To Canada: What Did The Canadian Supreme Court Say?
The top court said that while Kimberley's rights were in fact violated by Newfoundland's pandemic rules, these were within the reasonable limits. The court deemed the pandemic travels rules to be in bounds within the reasonable limits on rights and freedoms in Section 1 of the Charter.
Jessica Kuredjian, a lawyer at Cassels in Toronto, as reported by The Global Mail, said, "This is a great ruling, and an important one. It is very human case. It is a great example of where Charter rights impact the real rights of everyday citizens."
The ruling will serve as a precedent for the ambit of government actions and restrictions during potential health emergencies in the future.
In a majority ruling authored by Justices Andromache Karakatsanis and Sheilah Martin, the court noted that early pandemic deaths and scientific uncertainty created an “extraordinarily difficult situation,” requiring swift decisions to protect public health and prevent further loss of life.
The judgment marks the latest major court effort to define the balance between individual freedoms and government authority stemming from pandemic-era actions. Just last month, the Federal Court of Appeal found the federal government’s 2022 use of the Emergencies Act to curb large protests was not legally justified.
Friday’s ruling also adds to the legal interpretation of the 1982 Charter of Rights. While many Charter provisions have been heavily litigated over the years, Section 6 — mobility rights — has rarely been tested in court.
“There really was a dearth of jurisprudence on the topic,” said Anaïs Bussières McNicoll, director of the Civil Liberties Association’s fundamental-freedoms program.
The pandemic travel-restriction case effectively marks the Supreme Court’s first detailed examination of Section 6 as it applies to Canadians’ general freedom of movement within the country.
In their majority opinion, five judges stressed that Charter rights must receive “generous protection.” On mobility rights, they traced the concept back nearly a millennium, linking it to common-law traditions from the 1200s and even earlier “ancient customs.”
North London Measles Outbreak: 34 Cases Confirmed In Unvaccinated Children From Enfield
Credits: iStock
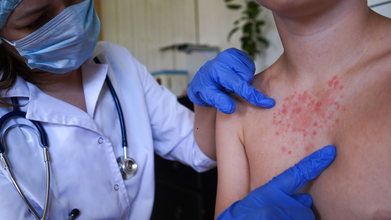
North London Measles Outbreak: 34 children have been infected by a "fast spreading" measles outbreak in several north London schools, confirmed health officials. The cases were first confirmed from Enfield in laboratory tests in January, as is reported by the UK Health Security Agency or the UKHSA.
A local GP, as reported by the BBC said that one in fiver children who contracted the illness had been admitted to hospital. The doctor also said that these children "had not been fully immunized".
Families are now asked to ensure that their children are up to date with their immunizations and vaccinations against this highly contagious disease. Measles could cause serious health complications.
North London Measles Outbreak: Who Can Get The Vaccines?
Measles vaccinations for children are available at the school, however, if they missed it, they can also get it at a number of catch-up clinics around the UK. The vaccinations are for free.
Enfield's NHS Ordnance Unity Centre For Health on its website noted that there is a "fast spreading measles outbreak in several schools" across the borough. The infections were confirmed in "at least" seven schools in Enfield, which means there could be more. Some reports also came from neighboring Haringey.
Enfield Councillor Alev Cazimoglu said that current outbreak had "mainly affected children and some have required additional care with a short stay in hospital". She also said, "Vaccination is the most effective way to protect yourself and your family. We urge everyone who is not fully vaccinated to act now."
The 34 cases of Enfield represent over a third of the 96 total cases which were confirmed in England in the first month of this year as per the UKHSA data.
As per the Enfield Council, it is working closely with UKHSA, the NHS and local partners to limit any further spread.
Read: UK Loses Measles Elimination Status: Why Is This Disease Making A Comeback?
North London Measles Outbreak: Who Are At Most Risk?
As per a UKHSA medical practitioner, Dr Vanessa Saliba, as also reported by the BBC, the "big" outbreak is "mostly affecting unvaccinated children under 10 in schools and nurseries". She also added, "Measles is a nasty illness for any child, but for some it can lead to long term complications and tragically death, but is so easily preventable with two doses of the MMRV [measles, mumps, rubella, chickenpox] vaccine."
Dr Saliba also suggested children to catch-up with their vaccine schedule in case they have missed it and also urged those travelling abroad over the Easter holidays to check their vaccination status.
North London Measles Outbreak: What Is It And What Are The Symptoms?
Measles is a highly contagious disease. It spreads by coughs or sneezes or by touching things that someone with measles has coughed or sneezed on.
Measles, also known as rubeola, is an extremely contagious viral illness that typically causes high fever, cough, runny nose, red and watery eyes, and a characteristic rash that begins on the face and spreads downward across the body. It spreads through respiratory droplets and can lead to severe and sometimes fatal complications, including pneumonia and inflammation of the brain known as encephalitis.
Symptoms include high fever, sore or red and watery eyes, coughing, sneezing, and small white spots in the mouth.
New Monkeypox Variant Emerges as Two Strains Combine, WHO Warns
Credit: Canva
Two strains of the monkeypox virus (MPXV) have combined with each other to create a new version of the disease, prompting the World Health Organisation to raise an alarm.
According to scientists, the Ib and IIb of MPXV have mutated together, and a case has been found in the UK and India, respectively. The first case was detected in the UK with travel history to a country in South-East Asia, and the second in India, with travel history to a country in the Arabian Peninsula.
Further analysis of each case shows that the two individuals fell ill several weeks apart with the same combined strain. Both cases had similar reported signs and symptoms of the disease and neither experienced severe outcomes.
As of now, these are the only known cases of this version of the virus.
What Is Mpox?
Mpox (formerly monkeypox) is an infectious disease caused by the monkeypox virus, a member of the Orthopoxvirus genus. Symptoms usually appear 1-21 days after exposure, and the illness lasts 2–4 weeks. People are considered to be contagious until all scabs have fallen off.
Mostly based in Africa, it was discovered by captive monkeys in 1958, after whom the disease was named in 1970. However, the name attracted many racist comments, especially on social media, where people wrote “the disease of monkeys” and associated it with Africans.
Under WHO guidelines, the naming of diseases must not drive any unnecessary negative impact on trade, travel, tourism or animal welfare, and avoid offending any cultural, social, national, regional, professional or ethnic groups. Thus, the name monkeypox became the ‘m-pox’.
How Can You Detect Mpox?
There are signs and symptoms of M-pox. They start to show within seven to 14 days of being infected. Therefore, for about a week, a person may not know they have m-pox, and they can spread it by travelling.
The earliest signs are getting a fever, sweating and having chills through your body. Other signs involve rashes, which start from a distant rash on the face and spread throughout the body. These rashes can be in different forms, sometimes a flat lesion, bumps, boils or scabs.
Symptoms can also include swollen lymph nodes, migraine, muscle aches, fatigue, weakness and back pain.
How Do You Prevent Mpox?
This is a contagious infection and can spread by skin-to-skin contact. Here are some things you can do to safeguard yourself:- Restrict your movement by avoiding going out in public, meeting people and interacting with animals.
- Wear clothes that will prevent skin-to-skin infections.
- Hydrate yourself to get rid of the toxins from your body
- Check if you have received your smallpox vaccination
Doctors also prescribe medications like acetaminophen and ibuprofen to treat the pain and fever one may experience after being infected
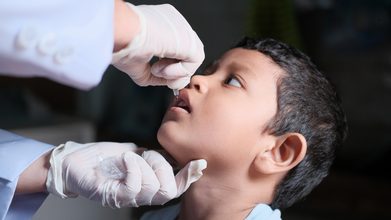
WHO Approves New India-Origin Oral Polio Vaccine for Outbreak Fight
Credit: Canva
The World Health Organization (WHO) has prequalified an additional novel oral polio vaccine type 2 (nOPV2) to strengthen the global supply of the vaccine, stop poliovirus type 2 outbreaks more sustainably and accelerate progress towards polio eradication.
The newly approved vaccine was manufactured by Biological E. Limited (BioE), Hyderabad, India, using in-house bulk vaccine following a technology transfer from PT Bio Farma (Persero), Indonesia.
The prequalification status means that the vaccine meets international standards of quality, safety, and efficacy for global immunization programmes.
It also allows the vaccine to be purchased and supplied through United Nations procurement agencies, including UNICEF, across the world and helps governments prevent and control poliovirus transmission.
With the new WHO recommendation, BioE is expected to produce 600 million doses per year of the nOPV2, increasing India's global leadership in vaccine manufacturing and its contributions to expanding access to affordable life‑saving vaccines across the Global South.
WHO Director-General Dr Tedros Adhanom Ghebreyesus highlighted the impact of vaccination efforts on global polio eradication and said: “Vaccines are also bringing us closer to the eradication of polio, with 41 cases of wild polio reported last year from just 24 districts in Pakistan and Afghanistan, down from 99 cases in 49 districts in 2024."
What Is nOPV2?
Novel oral polio vaccine type 2 (nOPV2) is a genetically modified, more stable vaccine designed to stop circulating vaccine-derived poliovirus type 2 (cVDPV2) outbreaks. It provides comparable protection to the old type 2 vaccine (mOPV2) but is less likely to cause new vaccine-derived outbreaks.
Studies show nOPV2 is safe, well-tolerated and produces similar immunity to mOPV2, with over 500 million doses administered across 23 countries as of early 2024. The vaccine is typically used in targeted, large-scale vaccination campaigns, often for children under five years of age.
The oral vaccine is supplied in 20-dose and 50-dose vial presentations. It has a shelf-life of 24 months when stored at temperatures not exceeding –20 °C and can also be stored for up to six months at +2 °C to +8 °C, providing important flexibility for immunization programmes in diverse situations.
Mike McGovern, Chair of the International PolioPlus Committee, Rotary International and Chair of the Polio Oversight Board, GPEI, said of the new prequalification: "Expanding nOPV2 manufacturing is essential to ensuring countries can respond quickly to variant poliovirus outbreaks. Biological E’s prequalification status strengthens the global supply and brings us closer to ending these outbreaks for good."
Why Is The New Polio Vaccine Useful?
Once a global scourge paralysing hundreds of thousands of children globally, polio is now close to eradication as wild poliovirus cases have fallen by more than 99 percent since the 1980s.
However, given rising vaccine hesitancy and gaps in immunisation, cVDPV2 can re-emerge in under-immunised communities, triggering outbreaks as seen recently in parts of Africa and Southeast Asia. But nOPV2's genetic stability and increased protection help limit that risk and reduce the chances of a mass outbreak.
While India achieved its polio-free certification in 2014, the risk of importations or vaccine-derived outbreaks increases if coverage drops. With the nOPV2's WHO prequalification, officials can safeguard public health by ensuring access to the most advanced tools available.
As of August 2022, 18 out of 21 countries had successfully stopped cVDPV2 transmission following two rounds of mass vaccination activity and a further two countries did so after a third round.
Data has shown that nOPV2 effectiveness is on par with that of mOPV2, and while evidence of the vaccine’s immunogenicity has already been well established, additional studies continue to be conducted throughout the EUL period to confirm protection against type 2 poliovirus in vaccinated individuals.
© 2024 Bennett, Coleman & Company Limited

