- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Who Is Susan Monarez? The New CDC Director Confirmed By The US Senate

Credits: AP/ CDC
In a closely watched vote, the U.S. Senate confirmed Dr. Susan Monarez as the new Director of the Centers for Disease Control and Prevention (CDC), marking a pivotal moment for an agency under intense political and public scrutiny. The 51-47 confirmation made Monarez the first CDC director to be Senate-confirmed under new legislation passed in 2023. The appointment follows months of leadership vacuum and ideological rifts inside the CDC that have fueled uncertainty in public health circles.
Monarez now steps into a role that has become as much about crisis management and restoring public trust as it is about public health science.
Who Is Susan Monarez?
At 50, Dr. Susan Monarez brings a formidable résumé steeped in science, biosecurity, and federal health policy. She holds a PhD in microbiology and immunology from the University of Wisconsin and completed her postdoctoral training at Stanford University. Before joining the CDC, Monarez served as Deputy Director at the Advanced Research Projects Agency for Health (ARPA-H), a government agency known for funding innovative biomedical research.
Her career spans multiple federal agencies including the Department of Homeland Security, the Health Resources and Services Administration, the Office of Science and Technology Policy at the White House, and the Biomedical Advanced Research and Development Authority (BARDA). These roles have given her deep exposure to health innovation, disaster preparedness, and pandemic response policy.
Also Read: Why Are Only Certain People At Risk Of Developing MS? World's First Study Aims To Crack The Code
Monarez has also led the development of national strategies to combat antibiotic resistance, close maternal health gaps, expand telehealth, and ensure the ethical use of health data. Her work has been consistently focused on bridging science and policy to tackle real-world health threats—both globally and domestically.
Monarez’s confirmation ends a stretch of instability at the CDC, which has been operating without a permanent director since early 2025. The agency has faced major staff losses, widespread morale issues, and increasingly vocal political pressure. President Trump’s initial nominee, David Weldon, was withdrawn unexpectedly, and Monarez was named acting director in January before being formally nominated in March.
During her confirmation hearing, Monarez maintained a diplomatic stance affirming her support for vaccines and science-based policy—while skirting direct questions about her relationship with Health Secretary Robert F. Kennedy Jr., whose controversial antivaccine positions have sparked upheaval within the agency. Kennedy has taken bold steps to sideline CDC authority, including bypassing the agency earlier this year to roll back COVID-19 vaccine guidance for pregnant women and children.
That move underscored the new reality Monarez inherits: a CDC whose power and relevance in setting public health policy is being redefined in real-time.
At her Senate hearing, Monarez was clear about her priorities: rebuilding public confidence in the CDC, modernizing outdated data infrastructure, and preparing for future infectious disease threats. These are not minor tasks. The CDC has suffered from a credibility gap over the last several years, exacerbated by mixed messaging during the COVID-19 pandemic, political interference, and what many in the public saw as a lack of transparency.
“She brings decades of distinguished experience in health innovation, disaster preparedness, global health, and biosecurity to CDC,” the agency said in a social media post following the vote. “Dr. Monarez will lead efforts to prevent disease and respond to domestic and global health threats.”
But restoring trust will take more than credentials. The CDC’s internal structure and external messaging need overhauling to match the expectations of a more skeptical and divided public. Monarez is taking over an agency in flux—both scientifically and politically.
Science and Strategy Behind the Leader
Monarez’s scientific work reflects a global and future-forward perspective. She has spent her career at the intersection of technology and health policy, especially in biodefense and medical preparedness. At ARPA-H, she helped direct funding toward tools for rapid disease detection, AI-driven health diagnostics, and innovative solutions for behavioral health and maternal care disparities.
She has also been deeply involved in policy development at the national level, helping craft presidential action plans and leading interagency coordination on issues ranging from pandemic preparedness to organ transplantation reform. In previous roles, she worked directly on combating multi-drug-resistant infections and led international collaborations with the EU, Canada, France, and others on biosecurity measures.
This blend of strategic vision and scientific rigor is exactly what public health experts say the CDC needs now—particularly in an era where emerging infectious diseases, misinformation, and technology disruptions often collide.
What’s Next for the CDC Under Monarez?
Monarez is stepping into the role amid mounting public health challenges. Vaccine skepticism is on the rise, federal funding for the CDC is set to be slashed under the 2026 budget proposal, and chronic disease burdens continue to grow alongside new threats like drug-resistant bacteria and climate-related health crises.
Her immediate task will be internal stabilization—building back teams, reaffirming scientific values, and reestablishing CDC leadership across state and local public health departments. Externally, she must walk a careful line: maintaining scientific integrity while navigating a politically volatile environment.
Already, her ability to operate in this tension-filled space is under watch. While her academic and professional pedigree earns her respect in science circles, her willingness—or reluctance—to directly challenge political directives from Secretary Kennedy and the White House will define her legacy at the CDC.
Susan Monarez’s confirmation is more than a personnel change; it signals a recalibration of how public health leadership will work in today’s polarized environment. Her track record suggests she understands the complexity and weight of the role.
The real test now is whether she can reassert the CDC’s voice in public health, without becoming another casualty in the ongoing tug-of-war between science and politics.
Flu Symptoms In Kids Could Be Deadly, Doctors Say Shot Is Still The Best Protection

Credits: Canva
The United States is in the middle of one of its worst flu seasons, according to the data by the Centers for Disease Control and Prevention (CDC). These cases are surging, along with an increase in hospitalization, driven by a mutated influenza A variant, H3N2, 'subclade K'. Experts have warned that flu activity could further increase in the weeks to come. Unlike past years, parents may have to jump through extra hoops to get their children a flu shot.
On January 5, the CDC dropped a long-held universal flu vaccine recommendation for kids 6 months and older. The recommendations stated that the shots should only be given after a discussion with a health care provider. This shift is feared by most experts as it could lead to a further decline in the number of people getting vaccinated. The shift has also come at the time when flu is hitting children hard, and at least 17 children have died from the flu season so far. The pediatric flu hospitalization rate is also the second highest for this point in the season in 15 years, reported NBC News.
Flu metrics have surpassed last season, as one of the deadliest for children in more than 20 years.
Pediatric Flu Deaths In 2024-25 Season
The 2024-25 flu season led to 280 flu-related pediatric deaths. This has been the highest number since the CDC started reporting these in 2004. Nearly all the children were unvaccinated.
Dr. Yvonne Maldonado, a pediatric infectious disease epidemiologist and professor at Stanford University, told TODAY.com, “It’s more than unfortunate; it's tragic," for the CDC to change its flu shot guidance for kids. “(Flu shots) probably are the most effective intervention in the last 100 years to reduce child deaths in this country.”
The change in the flu shot recommendation also drew criticism from the American Academy of Pediatrics and the Infectious Disease Society of America.
Experts have warned that the new guidance creates confusion, which could lead to fewer children getting vaccinated. However, doctors time and again have said that the best line of defense remains to be the flu shots. Dr Pedro Piedra, professor of molecular virology and pediatrics at Baylor College of Medicine told Today.com, "There is plenty of data showing the effectiveness... of the influenza vaccine in children." The CDC website also states that the "flu vaccine can prevent severe, life-threatening complications in children."
How Effective Is The Flu Vaccine?
The flu shot helps the immune system learn how to fight the virus by exposing it to harmless pieces of killed or weakened flu germs, allowing the body to build antibodies that can quickly recognize and stop the infection if exposed later.
While the flu shot may not always stop you from catching the virus, its biggest strength lies in making the illness far less dangerous. Vaccinated people are significantly less likely to be hospitalized, need intensive care, or die from flu-related complications.
Overall flu shot effectiveness usually falls between 40 to 60 percent, depending on the season, but studies show the protection is often stronger in children. A 2022 study cited by the CDC found that flu vaccination reduced the risk of severe illness in children by 75 percent. Another CDC-listed study from 2020 showed the vaccine lowered flu-related hospitalizations by 41 percent and emergency room visits by 50 percent during the severe 2017–2018 flu season. Earlier research from 2017 also found that flu vaccination cut a child’s risk of dying from the flu by up to 65 percent, even among otherwise healthy children.
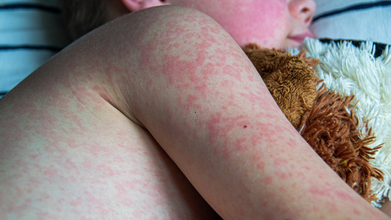
Kentucky Reports First Positive Measles Case of 2026: Confirmed Health Officials
Credits: Canva
Measles case, the first of 2026 is confirmed in Kentucky state, as the health officials announced the state's first positive measles case of the year on Thursday. The Kentucky Department for Public Health reports that an unvaccinated Jessamine County resident tested positive for measles. Officials have confirmed that the person was exposed to measles when an out-of0state travel who was infectious visited Fayette County between December 31, 2025 to January 2, 2026.
Previously, it was also reported by the health officials that the infectious person stayed at the Hyatt Place in Hamburg from December 31 and January 3. The person ate at Panera in Hamburg on January 1. This information will help in contract tracing.
Why Is Measles Making A Comeback In The US?
The measles outbreak in South Carolina started in October and from thereon, it showed no signs of slowing. In fact, Dr Linda Bell, South Carolina's state epidemiologist said at a news conference on Wednesday, this has happened because country's "lower-than-hoped-for vaccination coverage".
Read: Measles Warning: 'Be Careful, Holidays Worry Us', Says Doctor
Vaccination Rates Have Gone Down
In the 2024–25 school year, roughly 90 percent of students in Spartanburg County received all their required childhood vaccines, including the measles, mumps and rubella shot. While that number may sound high, it still falls short of the national average and the 95 percent coverage that experts say is needed to stop measles from spreading in a community.
Several of the schools where students are now in quarantine have vaccination rates that drop well below 90 percent, based on state data.
Health officials pointed out that measles can disrupt lives even for those who never get sick. In South Carolina, a few unvaccinated students were exposed to the virus twice, which meant they had to quarantine twice for 21 days each time. That is more than a month of missed school.
“That’s a significant amount of time,” Dr. Bell said. “Vaccination continues to be the best way to prevent the disruption that measles is causing to people’s education and to employment.”
State officials have increased their outreach around the MMR vaccine, although it is still unclear if these efforts are making a real difference. Uptake has been limited, according to Andrew Nixon, a spokesperson for the Department of Health and Human Services. He said vaccination remains “the best way to protect against measles” and encouraged people to speak with a doctor about what makes the most sense for them.
This is not just the case of South Carolina, but every where else the vaccination rates have fallen down, noted Harvard Health. In fact, same is the case with flu vaccines as rates have gone down, exposing more vulnerable population to such diseases.
Also Read: How Did This Once Eliminated Disease Come Back?
What Is Measles?
Measles, also known as rubeola, is a highly contagious viral illness that typically causes fever, cough, a runny nose, red and watery eyes, and a distinctive red, blotchy rash that usually begins on the face and spreads downward. The virus spreads through the air when an infected person coughs or sneezes and can lead to serious complications such as pneumonia or brain inflammation. Despite its severity, measles is preventable through a safe and effective vaccine, as per the Mayo Clinic.
How Contagious Is Measles?
Measles is among the most contagious diseases in the world. The virus spreads through airborne droplets that can linger in the air or on surfaces for hours. Up to 90% of unvaccinated people who are exposed to measles will become infected. A single infected person can pass the virus to an estimated 12 to 18 others through close contact or shared spaces. People can transmit the virus days before symptoms become obvious and continue spreading it after the rash appears, according to the World Health Organization.
How Long Is Someone Contagious With Measles?
Someone infected with measles can spread the virus from four days before the rash develops to four days after it appears. The virus spreads so efficiently that about 90% of people who are unvaccinated or have never had measles will become infected after being exposed.
In November, Canada lost its measles elimination status following a significant outbreak, according to the Pan American Health Organization, which works closely with the World Health Organization.
“It’s important to say that all the other 34 countries in the region, they keep their certification as measles-free,” said PAHO/WHO Director Dr. Jarbas Barbosa at the time, as per NPR News.
U.S. health officials have also warned that genetic links between outbreaks in different states suggest continued spread.
“The trajectory that we’re looking at now is that we do anticipate more cases well into January,” Bell said. “What that means for us nationally in terms of how they are defining our designation in this country as having eliminated measles is unclear.”
The Great Healthcare Plan Explained: What Is In President Trump's Health Care Proposal?

Credits: Wikimedia Commons
President Trump on Thursday launched The Great Healthcare Plan, a health care proposal that outlined a set of cost-cutting ideas. The White House website reads: "President Donald J. Trump is calling on Congress to enact the Great Healthcare Plan, a comprehensive plan to lower drug prices, lower insurance premiums, hold big insurance companies accountable, and maximize price transparency."
What Does This Great Healthcare Plan Offer?
In terms of lowering the price, prescribed drugs make it to the list, along with insurance premiums. Furthermore, the plan also states "holding big insurance companies accountable" and "maximize price transparency" as the features it will be introducing soon.
Also Read: Father Donates Liver To Save His One-Year-Old Son From A Rare Liver Disorder
Great Healthcare Plan By Trump: Lower Drug Prices
This allows lower prescription prices for all Americans by "codifying the Trump Administration's Most-Favored-Nation deals" to get Americans the same low prices for prescription drugs that people in other countries pay. This had already been introduced before on President's personal website, TrumpRx. On its launch, Trump signed deals with popular weight loss companies to lower their weight loss drug prices.
This initiative will further allow more over the counter medicines to be available at a cheaper rate. The website mentions that this will lower healthcare cost and increase "consumer choice by strengthening price transparency, increasing competition, and reducing the need for costly and time consuming doctor’s visits".
Great Healthcare Plan By Trump: Lower Insurance Premium
This feature includes three things:
- Send the Money Directly To The American People
- Fund cost-sharing Reduction Program
- Cut Kickback Costs
So, what do these mean? The first feature means that the plan aims to stop sending big insurance companies billions in extra taxpayer-funded subsidy payments. Instead, money will be sent directly to eligible Americans to allow them to buy the health insurance of their choice.
The second feature includes a cost-sharing reduction program for healthcare plans which would save taxpayers at least $36 billion and reduce the most common Obamacare plan premiums by over 10%.
Lastly, the plan aims to end kickbacks from pharmacy benefit managers to the large brokerage middlemen that deceptively raise the cost of health insurance.
Great Healthcare Plan By Trump: Hold Big Insurance Companies Accountable
The first feature 'Create the “Plain-English Insurance” Standard' means that it will require health insurance companies to publish rate and coverage comparison upfront on their websites in "plain English - not industry jargon". This will be done so that consumers can make better insurance purchasing decisions.
The plan will also require health insurance companies to publish the percentage of their revenues that are paid out to claims versus overhead costs and profits on their websites.
Lastly, it will require health insurers to publish the percentage of insurance claims they reject and average wait times for routine care on their websites.
Great Healthcare Plan By Trump: Maximize Price Transparency
The plan would require any healthcare provider or insurer who accepts either Medicare or Medicaid to
publicly and prominently post their pricing and fees to avoid surprise medical bills.
Why Is Trump's Great Healthcare Plan Is Being Introduced?
Earlier, polls have found that many voters were unhappy with the rising health care premiums and lawmakers' inability to reach a deal on ACA subsidies. This lapse led to a spike in health-insurance cost of millions of marketplace enrollees.
“There’ll be ongoing conversations, and we hope to be able to support with specific language for the legislation,” Mehmet Oz, one of Trump’s top health care deputies, told reporters Thursday on a conference call.
© 2024 Bennett, Coleman & Company Limited

