- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Indonesia Launches Mass Vaccination Drive After Deadly Measles Outbreak

Credits: Canva
Hundreds of children lined up at health centers in East Java on Monday as Indonesia began a mass vaccination drive to curb a deadly measles outbreak that has claimed 17 lives in recent months. The campaign, which targets tens of thousands of young children, comes amid concerns over low vaccination coverage in the world’s fourth most populous nation.
Outbreak Hits East Java Hard
According to the Sumenep District Health Agency, more than 2,000 children have been infected with measles in East Java over the past eight months. Sixteen of the 17 deaths were reported in Sumenep district, where officials revealed that none of the patients had received complete measles immunization.
Deputy chief of Sumenep district Imam Hasyim urged parents to ensure their children receive the shots. “Otherwise, this disease, measles, will spread further among our children. It will be even more fatal in the future,” he said. Authorities are aiming to vaccinate 78,000 children aged between nine months and six years as part of the emergency drive.
What Is Measles?
Measles is a highly contagious viral disease that spreads through coughing, sneezing, or direct contact with an infected person. Symptoms often start with a high fever, cough, runny nose, and red eyes, followed by a distinctive red rash that spreads across the body.
While measles may seem like a childhood illness, it can cause severe complications such as pneumonia, brain inflammation, blindness, and even death. The World Health Organization (WHO) warns that measles is among the leading causes of death among young children globally, despite the availability of a safe and effective vaccine.
Past Outbreaks in Indonesia
This is not Indonesia’s first struggle with measles outbreaks. In 2018, the eastern province of Papua experienced a major outbreak that killed dozens. At the time, vaccination efforts faced hurdles when the Indonesian Ulema Council announced that the measles-rubella vaccine contained pork derivatives. While authorities permitted temporary use of the vaccine made by India’s Serum Institute, vaccination rates dropped as debates over halal certification grew.
Last year, only 72% of Indonesia’s 22 million children under the age of five received the measles vaccine, according to government statistics. In some provinces, coverage fell below 50%, far short of the 95% rate needed to prevent outbreaks.
Global Concern Over Measles Resurgence
The outbreak in Indonesia mirrors a troubling global trend. WHO reported that 60 countries experienced major measles outbreaks in 2023. Worldwide, about 84% of children received their first dose of the vaccine and 76% got the recommended second dose. Public health experts, however, stress that vaccination rates must be significantly higher to achieve herd immunity.
In the United States, the Centers for Disease Control and Prevention (CDC) recently reported the highest number of measles cases in over 30 years. Similar outbreaks have been recorded across Europe, Africa, and Latin America, underscoring how quickly the virus can spread when immunization coverage declines.
Authorities Call for Collective Action
Indonesian officials have appealed to local leaders, community groups, and religious authorities to encourage participation in the vaccination campaign. Given the sensitivity around vaccine ingredients in the past, their role is considered vital in restoring public trust.
Health experts stress that strengthening immunization programs is critical for Indonesia’s future. With millions of children still vulnerable, they warn that outbreaks will continue unless vaccine coverage improves substantially.
As the latest campaign rolls out, parents like those in Sumenep are being urged to prioritize their children’s protection. For many, this mass drive could mean the difference between life and death.
Can You Test Negative For Covid And Still Be Infected?
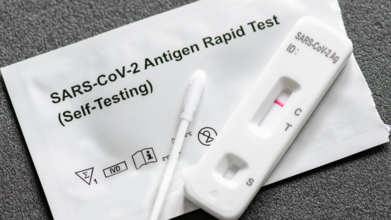
Credits: iStock
You wake up with a scratchy throat, a runny nose, and a nagging cough, but your home COVID-19 test comes back negative. How can this be? The short answer is yes—you might be infected. Lots of other viruses such as influenza are being passed around at high levels in the US, so it is getting harder and harder to tell if someone has COVID-19 or another infection based on how they're feeling.
The Centers for Disease Control and Prevention (CDC) is clear that a negative result is not always a sign of absence of infection. The viral loads can be too low to be detected at first, and timing of testing, sampling, and test sensitivity all become important factors. For patients with symptoms, the CDC advises consideration of further tests, including PCR tests, that are more sensitive than rapid antigen tests.
Yes, you can be infected with COVID-19 and test negative. This typically occurs when the viral load is below the detectable level at the time of the test, if the sample was not taken correctly, or if the test performed (such as a rapid antigen test) is less sensitive than PCR. Being in the early stages of the infection, having had a previous immunity, or having differences in viral shedding can all give rise to negative results even though you are contagious. Repeating the test after a day or two, or using a PCR test, increases detection accuracy.
Can COVID Tests Can Fail Early On?
Lateral flow tests (LFTs), commonly applied for quick detection, are most precise when viral levels are high. In early infection or in already immunized individuals due to vaccination or previous infections, viral levels can be low, leading to negative test results while being actively infected. Immune systems become activated quicker in previously infected persons, causing symptoms before the virus becomes detectable.
Virologist describe how SARS-CoV-2 shows varied behavior in the human body. Some people have peak viral loads that are thousands of times larger than those of other people, and others clear the virus within a matter of days. This variability is the reason why symptoms alone do not always match infectiousness.
What is The Role of PCR Testing and Medical Guidance?
PCR tests in clinical environments are more sensitive than home antigen testing and can identify infections sooner. Medical professionals can further distinguish COVID-19 from other viral diseases, enabling timely treatment and counseling. Early diagnosis is still crucial, particularly among high-risk groups or those with comorbidities.
What Causes A Negative Test?
There are various reasons for a negative test result:
- Early testing prior to adequate viral replication
- Incorrect sampling or swabbing method
- Partial immunity from vaccination or earlier infection leading to low viral loads
- Infection with a different virus that has overlapping symptoms with COVID-19
It is also conceivable to be infected with several different viruses at once, making it even harder to interpret. Specialists advise retesting a day or two after an initial negative test if symptoms have not gone away, instead of trying to rely on one negative test result.
What Is The Isolation Period?
With the variability of viral shedding patterns, it's not easy to pinpoint the moment when an individual is contagious. The CDC's revised Respiratory Virus Guidance recommends individuals remain at home at least 24 hours after symptom improvement and fever abatement without the use of medicine. Additional precautions like masking, better ventilation, and distancing for five more days are suggested to reduce the risk of transmission.
Serial testing—having several tests over a period of days—can provide a better image of viral activity. Successive negatives after earlier positives can signal decreased infectiousness, but the timing and kind of test are important factors.
Effective Tips To Keep In Mind for Summer Covid-19 Surge
If symptoms develop but are negative on rapid tests, PCR testing for confirmation should be considered.
Watch carefully for symptoms, and have medical assessment to exclude other viral infections.
Follow CDC recommendations for isolation and after-recovery precautions to safeguard others.
Take note of local virus circulation patterns, as several respiratory viruses may be circulating at the same time.
COVID-19 testing, while necessary, is not without its limitations. Infection may not be excluded by negative test results, particularly during the early stages of infection or in the presence of widespread viral transmission. It is essential to be aware of overlapping respiratory pathogens, timing of testing, immune history, and variability in viral load to accurately interpret results. As SARS-CoV-2 continues to co-circulate with influenza, RSV, and norovirus, caution should be exercised, sensitive tests employed where indicated, and revised isolation and prevention measures followed.
What this indicates is that your test can be negative, yet your immune system's reaction and viral dynamics are something else. The best here is vigilance, frequent re-testing where possible, and adhering to public health recommendations to care for yourself and others.
Six Secret Triggers That Could Speed Up Your Ageing Process Faster Than Others, Scientists Reveal

Credits: Canva
Age is a universal truth—every second that passes, we move closer to our later years. But growing older and ageing aren’t exactly the same. Ageing refers not merely to the passage of time but to how our bodies and minds cope with it. Intriguingly, some people maintain vitality and health well into their 80s or 90s, while others experience frailty, cognitive decline, or chronic illnesses far earlier.
A recent study led by an international team at the University of Colorado Boulder sheds light on why this happens. Published in Nature Genetics, the research identifies six distinct biological pathways that accelerate ageing, revealing how genetics and lifestyle combine to influence our longevity and overall health.
What Accelerates Unhealthy Ageing In Human Beings?
Frailty, defined as multisystem physiological decline, is a hallmark of unhealthy ageing. In the United States, more than 40% of adults over 65 are considered frail, showing symptoms like slower walking speed, weaker grip, and a higher number of diagnosed illnesses. But frailty isn’t uniform. Two people with identical frailty scores could be struggling with entirely different issues—one might face mobility limitations, while the other battles cognitive decline.
Dr. Kenneth Rockwood, a frailty expert at Dalhousie University in Canada and co-author of the study, emphasizes that ageing is not a single process. “Aging is not just one thing. There are many ways to be frail. The question then becomes: What genes are involved?”
To tackle this question, researchers examined DNA and health data from hundreds of thousands of participants in the UK Biobank, mapping 408 genes linked to 30 frailty symptoms. This was a dramatic increase from the 37 genes previously associated with accelerated ageing, revealing a much broader genetic landscape than previously understood.
What Are The Six Triggers That Can Make You Age Faster?
The study highlights six distinct pathways that contribute to unhealthy ageing, each with its own underlying biology:
Disability-Linked Ageing: Genes affecting mobility, coordination, and physical strength. Individuals in this group often experience reduced walking speed, poor balance, and difficulty performing daily tasks.
Cognitive Decline: Genes influencing brain function, memory, and learning. Those affected are more likely to develop dementia, Alzheimer’s disease, or other forms of cognitive impairment.
Metabolic Dysfunction: Genes involved in metabolism and energy regulation. This pathway can result in obesity, insulin resistance, or diabetes, accelerating age-related deterioration.
Multiple Disease Burden: A combination of genetic predispositions that increase susceptibility to several chronic conditions simultaneously, including cardiovascular disease, arthritis, and cancer.
Unhealthy Lifestyle: Environmental and behavioral factors such as poor diet, smoking, sedentary habits, and insufficient sleep, which interact with genetic susceptibility to exacerbate ageing.
Limited Social Support: Genes and psychosocial factors that influence mental health, stress response, and social engagement. Lack of social networks can compound physical and cognitive decline.
Why Do Some People Get Better With Age?
Senior author Andrew Grotzinger, assistant professor of psychology and neuroscience at CU Boulder, highlights that while the study focuses on genetic underpinnings, lifestyle factors cannot be ignored. “This paper not only identifies sub-facets of disordered aging but also demonstrates that there is very different biology underlying them,” he explains.
For example, someone with genetic vulnerabilities in metabolism may mitigate accelerated ageing by maintaining a balanced diet and regular exercise, while social isolation may amplify cognitive and physical decline in another individual. Understanding the intersection of genes and lifestyle is critical for designing personalized interventions.
This study represents the largest genetic exploration of frailty to date, providing a roadmap for future interventions aimed at slowing or reversing unhealthy ageing. By identifying the distinct subtypes, researchers hope to develop targeted therapies that address the root biological causes rather than just the symptoms of frailty.
Potential interventions could include cognitive training programs for those genetically predisposed to mental decline, metabolic regulation therapies, or community-based programs to improve social engagement and psychological resilience.
Moreover, the findings could inform public health strategies, helping clinicians identify at-risk populations earlier and provide tailored guidance on diet, exercise, and social habits.
While ageing is inevitable, unhealthy ageing is not. The insights from this study could revolutionize how we approach longevity, offering the possibility of personalized ageing plans based on genetic profiling and lifestyle interventions.
As Dr. Isabelle Foote, co-author and postdoctoral researcher at CU’s Institute for Behavioral Genetics, notes, “To be able to identify treatments to stop or reverse accelerated biological aging, you need to know what the underlying biology is. This is the largest study yet to use genetics to try to do that.”
By recognizing the six distinct triggers of accelerated ageing, scientists are laying the foundation for therapies that could help millions of people maintain health, mobility, and cognitive function well into their later years.
This Thing In Your Hair Could Heal Your Tooth Enamel And Keep Them Pearly White

Credits: Canva
The search for stronger, longer-lasting teeth has been a cornerstone of dentistry. From the widespread use of fluoride to modern resin fillings, the field has steadily advanced but has never managed to replicate the extraordinary natural material that coats our teeth: enamel. Once it erodes, it’s gone forever—or at least, that’s what we’ve always believed.
Now, researchers from King’s College London are challenging this assumption with an unusual but promising source: keratin, the protein that makes up human hair and animal wool. Their findings suggest that something as simple as a haircut could one day contribute to regenerating tooth enamel and transforming oral care.
Why Tooth Enamel Is Irreplaceable?
Tooth enamel may look simple—a hard, shiny coating that gives teeth their strength and luster—but it is one of the most remarkable substances in the human body. Harder than bone, enamel is designed to withstand decades of grinding, chewing, and exposure to temperature extremes.
Unlike bone, however, enamel is non-living. It lacks the cells and blood supply necessary to heal itself. That’s why a small cavity or a patch of erosion, if left untreated, can become a permanent problem. Once enamel wears away, it exposes dentin, a softer layer that appears yellow and is far more vulnerable to decay.
The impact of enamel erosion is staggering. Dental decay weakens a tooth’s strength by up to 95 percent, leaving it prone to fractures, sensitivity, and eventually loss. According to the Global Burden of Disease 2019, untreated cavities affect an estimated two billion people worldwide, making dental decay the most common disease on the planet.
What Are The Limitations of Current Dental Treatments?
Modern dentistry has developed tools to slow or mask the damage caused by enamel loss, but not to restore it. Fluoride can strengthen remaining enamel and delay erosion, but it cannot rebuild what has already vanished. Resin-based fillings, while effective in patching cavities, are no match for the natural durability and resilience of enamel. Worse still, resins can contain toxic compounds and lack the long-term strength of natural tooth material.
The result is a cycle of temporary fixes. Cavities are filled, fillings fail, larger restorations follow, and eventually, teeth are lost. As populations age and diets grow increasingly sugar-heavy, the financial and health burden of this cycle is enormous. The challenge has been clear: how can dentistry move beyond patchwork solutions to true biological regeneration?
How Does A Hair Protein Improve Your Teeth?
The answer may lie in keratin, the fibrous protein best known for forming hair, nails, and wool. In their study, researchers at King’s College London extracted keratin from sheep wool and introduced it into a solution designed to mimic human saliva. To their surprise, the keratin didn’t simply dissolve or degrade—it began pulling minerals from the artificial saliva and assembling them into structures that closely resembled natural tooth enamel.
The regenerated material didn’t just look like enamel under a microscope; it behaved like enamel, too. It demonstrated the same stiffness, resistance to wear, and pearly shine that makes natural teeth so resilient.
What’s more, the team discovered that mixing different types of keratin produced superior results. By layering proteins in a hierarchical structure—similar to Russian nesting dolls—they achieved enamel-like material with remarkable strength, durability, and resistance to various forms of degradation.
Attempts to regrow enamel are not new. Previous efforts have focused on peptides, stem cells, and synthetic biomaterials. Yet these approaches have often stumbled over practical barriers, from poor bioavailability to the inability to repair deep cavities.
Keratin may offer a way around these roadblocks. It is abundant, renewable, and can be sourced from waste materials like wool or hair, aligning with a circular economy model. Unlike synthetic resins, keratin-based materials are biocompatible and less likely to trigger toxicity or rejection.
As Dr. Sherif Elsharkawy, the study’s senior author, put it: “We are entering an exciting era where biotechnology allows us to not just treat symptoms but restore biological function using the body’s own materials.”
While the concept might sound futuristic, researchers believe keratin-based enamel boosters could be available within two to three years. Potential applications range from everyday products like toothpaste to more targeted dental gels applied by clinicians.
Imagine visiting your dentist not for a drill-and-fill appointment but for a keratin “varnish” that coats your teeth, hardening over time into new enamel. Or brushing daily with a toothpaste that rebuilds microscopic enamel loss before it develops into a cavity.
If successful, these products could revolutionize dental care by shifting the focus from repair to regeneration.
The implications stretch far beyond whiter smiles. Dental decay is a leading cause of pain, disability, and lost productivity worldwide, especially in low-resource settings where access to dental care is limited. A safe, affordable way to restore enamel could dramatically reduce the burden of oral disease across populations.
The study, published in Advanced Healthcare Materials, is still in early stages. Researchers must test keratin-based enamel in real-world conditions, ensuring it can withstand the stresses of chewing, exposure to bacteria, and years of daily use. Clinical trials will be critical before any commercial rollout.
Still, the concept is generating excitement as co-author Elsharkawy noted, “With further development and the right industry partnerships, we may soon be growing stronger, healthier smiles from something as simple as a haircut.”
© 2024 Bennett, Coleman & Company Limited

