- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Your Blood Type May Influence Your Risk Of Early Stroke

Credits: Canva
People with type A blood may have a higher risk of suffering a stroke before the age of 60 compared to those with other blood types, according to a 2022 study published in Neurology. The research, which involved a comprehensive analysis of genetic data, highlights a possible connection between blood type and early-onset ischemic stroke—a type caused by reduced blood flow to the brain.
Large-Scale Genetic Analysis
Researchers from around the world compiled and reviewed data from 48 genetic studies, including nearly 17,000 individuals who had experienced a stroke and about 600,000 controls who had not. All participants were between 18 and 59 years of age. By conducting a genome-wide association study (GWAS), they aimed to identify genetic variants linked to early stroke.
Their search revealed two genetic regions significantly associated with stroke risk. One of them was located near the genes that determine blood type. A closer analysis showed that people with a genetic variant coding for a subtype of type A blood—specifically the A1 subgroup—had a 16% higher chance of having a stroke before turning 60 compared to those with other blood types.
On the other hand, individuals with a gene variant linked to blood group O1 had a 12% lower risk of early-onset stroke.
Why Blood Type Might Matter
While the observed increase in stroke risk for people with blood type A is modest, the finding raises important questions about underlying biological mechanisms.
“We still don't know why blood type A would confer a higher risk,” said study co-author Dr. Steven Kittner, a vascular neurologist at the University of Maryland. “But it likely has something to do with blood-clotting factors like platelets and cells that line the blood vessels as well as other circulating proteins, all of which play a role in the development of blood clots.”
Since strokes in younger individuals are less often caused by long-term artery damage like atherosclerosis and more frequently due to clot-related issues, researchers believe that blood type might influence these clotting mechanisms more strongly in early-onset cases.
Differences Between Early and Late-Onset Stroke
To explore whether the relationship between blood type and stroke was age-dependent, the team also compared genetic data from another 9,300 people who had a stroke after age 60 with 25,000 older adults without a stroke.
Interestingly, they found that the link between blood type A and stroke became statistically insignificant in the older group, suggesting that strokes occurring earlier in life may have a distinct biological pathway.
Other Findings
The study also found that individuals with type B blood had about an 11% increased risk of stroke compared to non-stroke controls, regardless of age.
Previous research has already shown that the ABO gene region—responsible for determining blood type—is associated with increased risks of coronary artery disease, heart attacks, and venous thrombosis (blood clots in the veins). These findings further support the idea that blood type may play a role in how the body forms and dissolves clots.
It’s worth noting, however, that the participants in this study were primarily of European ancestry, with only 35% from non-European backgrounds. The researchers emphasize the need for more diverse studies to understand the broader implications of blood type on stroke risk across populations.
Despite the findings, experts caution against unnecessary alarm. “The increased risk is relatively small, and people with type A blood shouldn’t be overly concerned,” said Kittner. “There’s no recommendation at this point for extra screening or preventive action based on blood type alone.”
Still, the research opens the door to further exploration of how inherited traits like blood type could contribute to stroke risk and potentially inform more personalized approaches to prevention and treatment.
Why Stopping Antidepressants Can Be Harder, Here's How Withdrawal Could Last Longer Than You Think

Credits: Canva
For years, stopping antidepressants was considered a relatively minor medical event—just a temporary adjustment phase. Official guidelines, particularly in the UK and the US, once described withdrawal symptoms as “brief and mild.” But now, emerging research is painting a very different picture—one that could affect millions of long-term users.
Recent findings suggest that withdrawing from antidepressants, especially after prolonged use, can trigger severe and long-lasting symptoms, sometimes lasting months or even years. Despite mounting evidence, some recent reviews—funded in part by pharmaceutical ties—continue to rely on outdated, short-term studies that don’t reflect the real-world experiences of long-term patients.
The modern class of antidepressants—SSRIs and SNRIs—were introduced in the 1980s and ’90s. When regulatory guidelines were first drafted, they leaned heavily on industry-funded clinical trials, where participants had taken the medications for only 8 to 12 weeks.
Because withdrawal symptoms in those short-term trials were limited and often transient, major healthcare bodies like NICE (National Institute for Health and Care Excellence in the UK) assumed the same would hold true for all patients.
That assumption, however, ignored the experiences of millions of people who were on antidepressants for much longer periods.
A comprehensive new study of NHS patients in the UK has shed light on the scale and severity of antidepressant withdrawal. The findings reveal that patients who had been on antidepressants for more than two years were ten times more likely to experience withdrawal symptoms compared to those who had taken the medication for less than six months. Moreover, the likelihood of experiencing severe symptoms increased fivefold in long-term users. Perhaps most strikingly, symptoms that lasted for three months or more were found to be 18 times more common among those who had used antidepressants for extended periods.
In contrast, those who took antidepressants for six months or less mostly experienced mild and short-lived symptoms. Around 75% reported mild or no withdrawal, and only one in four had difficulty stopping but for long-term users, it’s a different story altogether. Two-thirds reported moderate to severe withdrawal symptoms, and nearly a third had symptoms that persisted for over three months.
In some cases, withdrawal effects were so debilitating that patients sought emergency care.
Against this backdrop, a newly published review in JAMA Psychiatry is drawing criticism. The review—which includes authors with known financial ties to pharmaceutical companies—relied on 11 short-term studies, most involving people who took antidepressants for 8 to 12 weeks. Only one study included participants on the medication for more than six months.
Not surprisingly, the review concluded that withdrawal symptoms from antidepressants are not clinically significant. It even went so far as to suggest the symptoms might be caused by the “nocebo effect”—the idea that negative expectations can create physical symptoms.
But experts argue that this reasoning is flawed and potentially harmful. The review failed to account for long-term users, excluded several studies with high withdrawal rates, and assumed that common symptoms like dizziness or fatigue are indistinguishable from genuine withdrawal effects.
Another glaring issue? The authors treated withdrawal symptoms reported by people stopping a placebo as equivalent to those reported by people stopping real antidepressants—despite clear evidence that the intensity, duration, and impact of symptoms differ significantly between the two. According to recent investigations:
- In England, 2 million people have been taking antidepressants for over five years.
- In the United States, that number is at least 25 million.
That’s tens of millions of people whose withdrawal experiences are underrepresented in medical literature and underserved by health systems.
To use an analogy, relying on eight-week antidepressant studies to predict withdrawal is like testing car safety at 5 km/h, when most people drive at 60 km/h. It simply doesn’t capture the full risk.
Is Antidepressant Withdrawal A Real Thing?
Yes, antidepressant withdrawal is real—especially if you stop the medication abruptly after taking it for more than four to six weeks. These symptoms, also known as antidepressant discontinuation syndrome, can last for several weeks and vary depending on the type of antidepressant.
Importantly, experiencing withdrawal doesn’t mean you’re addicted. Addiction involves compulsive use, cravings, and harmful consequences—none of which apply to antidepressants.
To avoid uncomfortable withdrawal effects, always consult your doctor before stopping. Most healthcare providers recommend gradually tapering the dose over weeks or months to help your body adjust. In some cases, a temporary switch to another medication might be advised to ease the transition.
If you're switching to a new antidepressant, your doctor may overlap medications to prevent withdrawal symptoms.
Since withdrawal can mimic a relapse of depression, it's essential to stay in close contact with your healthcare provider. If symptoms of depression return, your doctor may suggest restarting treatment or exploring alternative therapies.
Always follow a medical plan when discontinuing antidepressants—never stop them on your own.
What Withdrawal Feels Like?
Withdrawal symptoms can vary, but often include:
- Dizziness or “brain zaps”
- Nausea
- Headaches
- Anxiety and panic
- Mood swings
- Insomnia
- Irritability
- Sensory disturbances (like sensitivity to light or sound)
For some, these symptoms are manageable. But for others—especially those stopping medication after years of use—they can be overwhelming and life-altering.
Withdrawal from antidepressants is real, and it’s often far more difficult than current medical literature suggests—especially for long-term users. Reviews that lean on short-term, pharmaceutical-funded data do little to help the millions of people struggling silently.
Mental health treatment should never be a one-way street. People deserve a clear roadmap for both starting and stopping antidepressants safely, compassionately, and with evidence-based support.
This Man Miraculously Beat Cancer Thrice; One Was Diagnosed Due To A Strange Symptom He Experienced During Cycling

(Credit-American Cancer Society)
After several years, multiple diagnosis and cancer treatments, Steve Drayton continues to give back to his community and those who stood by him even during tough times. Not only was he diagnosed with HIV, or Human Immunodeficiency Virus which attacks the body's immune system, but he also survived cancer three times. Going through all of these harrowing processes did not make him disheartened, instead this only fueled his drive to support and advocate for the well-being and health gap experienced by LGBTQIA+ community through education and advocacy.
Promise Made Amidst the HIV Epidemic
In the 1990s, when the HIV epidemic was devastating the gay community, Steve personally experienced its harsh reality. In 1994, he was diagnosed with Kaposi sarcoma. KS is a type of cancer that is often seen in people with weakened immune systems. Being diagnosed with HIV, his immune system was already compromised and this diagnosis confirmed that his HIV had progressed to AIDS.
What is Kaposi Sarcoma (KS)?
Kaposi sarcoma (KS) is a type of cancer that usually shows up as purple spots or bumps on your skin, in your mouth, or in your digestive system. These growths can also spread to other parts inside your body. It's caused by a specific virus called human herpesvirus 8 (HHV-8). However, most people who have this virus do not get KS. This cancer usually develops in people whose immune system is weak, like those with HIV, individuals who have received an organ transplant, or older adults.
Steve and his best friend both received HIV and AIDS diagnoses. Sadly, his friend did not survive. Before his friend passed away, Steve made a heartfelt promise: he would do everything he could to prevent others from enduring the same suffering.
Honoring this promise, Steve immediately took action. He began raising money for HIV resources, joined support groups, and taught safe sex classes in prisons to educate people on how to reduce their risk of HIV. He credits his best friend as the inspiration for his lifelong journey of advocacy.
Bike Ride Leads to a Life-Saving Discovery
Years later, in 1999, Steve met his husband, Stephen. Together, "the Steves" expanded their advocacy efforts. Witnessing many friends and family affected by cancer, they found a shared passion for cycling and began participating in bike rides to raise money for cancer research.
In 2016, one particular bike ride dramatically changed Steve's life. While participating in the American Cancer Society Bike-A-Thon, riding from Philadelphia to Ocean City, New Jersey, Steve felt an unusual discomfort. It was the morning after the tragic Pulse nightclub shooting, and despite the emotional weight and physical exertion of the ride, he knew something was wrong with his body.
Steve reported his symptoms to his doctor, leading to a quick colonoscopy. In September 2016, at age 56, he was diagnosed with Stage III rectal cancer. The National Institute of Cancer explains that this kind of cancer forms in the rectal tissue of the rectum. He believes the discomfort he felt while cycling, which prompted him to see a doctor, was crucial in catching the cancer early.
He underwent two months of chemotherapy and radiation. While his doctors warned of side effects, Steve was unprepared for the intense and persistent rectal pain. To find relief, he opted for an ostomy procedure, which created a new way for waste to leave his body. The pain subsided, but Steve had to adjust to permanently wearing an ostomy pouch. In July 2018, Steve received his third cancer diagnosis: squamous cell skin cancer. He was treated with surgery and has had no recurrence, continuing annual skin checks.
Understanding Squamous Cell Skin Cancer
Skin cancers typically begin in the epidermis, the outermost layer of your skin. This layer has two main types of cells where cancer can start. Squamous cells are the flat cells on the surface that are always shedding. When these grow abnormally, they can become squamous cell skin cancer. Below them are basal cells, which constantly divide to create new skin cells. As these new cells move upward, they flatten out and become squamous cells. When cancer begins in these basal cells, it's called basal cell skin cancer.
Treatment And Advocacy For Multiple Cancer
Nearly seven years after his skin cancer diagnosis and ten years after his rectal cancer diagnosis, Steve is living cancer-free. He and Phen are approaching their 26th anniversary and still enjoy their Saturday date nights. Although treatment effects prevent Steve from cycling now, he and Phen continue to be active advocates in their community. They share their powerful story, encouraging everyone to be aware of their cancer risks and to get recommended screenings. Steve believes it's his duty to share his journey and raise awareness about organizations like the American Cancer Society.
Olivia Munn’s Mom Diagnosed With Breast Cancer After Actor Urges Her To Take Risk Test; What Should You Do If It Runs In Your Family?
Credits: Instagram/@OliviaMunn
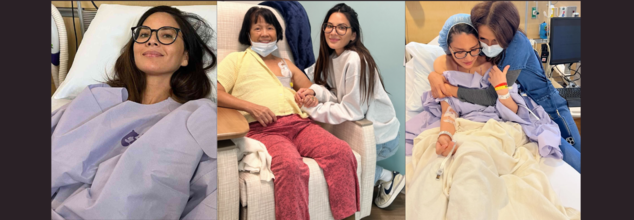
Actress Olivia Munn, who has spoken candidly about her 2023 breast cancer diagnosis, shared a powerful new development: her mother, Kim, was also diagnosed with breast cancer after taking the same risk assessment test that led to Munn’s own diagnosis.
In an Instagram post, Munn said her mother was diagnosed with Stage 1 HER2-positive breast cancer, a fast-growing but often treatable type of the disease. Her diagnosis came after Munn encouraged her mom and sister to take a free online breast cancer risk assessment, known as the Tyrer-Cuzick Risk Model.
The tool, which is publicly accessible and widely recommended by experts, calculates a woman’s five-year and lifetime risk of developing breast cancer using a combination of factors like age, family history, genetics, reproductive history, and more.
“My mom scored 26.2%. Her yearly mammogram had just come out clear, but because of that high score I insisted she get an MRI,” Munn wrote. That MRI led to her mother’s diagnosis—despite no red flags on her recent mammogram.
Since then, Kim has completed 12 rounds of chemotherapy, and Munn says she will continue monthly Herceptin infusions until the fall. Kim also underwent a double mastectomy, just like her daughter did.
Munn’s social media post included photos and videos of her mother receiving treatment, preparing meals after surgery, and eventually ringing the bell after her last chemo session, surrounded by hospital staff holding signs.
“She’s handled all of this with bravery and humor,” Munn wrote, adding that her mom insisted on cooking and doing laundry days after surgery. “She’s insane—but I’m so proud of her.”
What Is the Tyrer-Cuzick Breast Cancer Risk Test?
The Tyrer-Cuzick model, also called the IBIS Risk Evaluator, is a mathematical tool used by healthcare professionals to estimate a woman’s likelihood of developing breast cancer. It’s based on clinical and personal information, such as:
- Family history of breast and ovarian cancer
- Age and age of first period
- Menopausal status
- Breast density
- Genetic markers (like BRCA mutations, if tested)
This model is often used alongside other screening tools to determine whether a woman might benefit from additional imaging like MRI scans or preventive treatments.
According to the National Cancer Institute, a lifetime risk over 20% is considered high, and patients in this category may need more aggressive or frequent screening than standard mammograms alone.
Why Mammograms Aren’t Always Enough For Breast Cancer Detection?
One of the most startling takeaways from Munn’s story is this: Kim’s mammogram had come back clean. It was only after reviewing her Tyrer-Cuzick score and following up with a breast MRI that the cancer was detected.
This is not uncommon. Mammograms are essential but not foolproof. They can miss up to 20% of breast cancers, particularly in women with dense breast tissue.
For women at higher risk, breast MRIs offer greater sensitivity and can detect tumors that mammograms might miss.
When Cancer Runs in the Family, What How Genetics Increase Risk?
Munn’s story highlights a critical issue—hereditary risk, and how understanding it can help catch cancer early or even prevent it. Her case, and her mother’s, raise an important question:
Can Breast Cancer Be Inherited from a Parent—and What Should You Do If It Runs in Your Family?
Yes, breast cancer can absolutely run in families. According to the American Cancer Society, about 5% to 10% of breast cancers are hereditary, meaning they're caused by gene mutations passed down from a parent.
The most well-known mutations are in the BRCA1 and BRCA2 genes, which significantly increase the risk of both breast and ovarian cancer. But other genes—such as PALB2, CHEK2, and ATM—have also been linked to elevated breast cancer risk. Here’s what to keep in mind if you have a family history:
1. Know Your History
If your mother, sister, grandmother, or even male relatives had breast or ovarian cancer—especially before age 50—your risk may be elevated. Multiple family members with cancer, or cases of both breast and ovarian cancer in one family, are red flags.
2. Get a Risk Assessment
Tools like the Tyrer-Cuzick model help calculate your personal risk level using both genetic and lifestyle factors. Many healthcare providers use it to guide recommendations for further testing or screening.
3. Consider Genetic Counseling
If your risk appears high, a genetic counselor can help you decide whether to undergo genetic testing. This is especially useful if you’re unsure of your family’s medical history or come from an ethnic background with higher BRCA mutation prevalence (e.g., Ashkenazi Jewish).
4. Screening and Prevention
If you’re identified as high-risk, you may be advised to begin annual MRIs and mammograms starting earlier than age 40. Some women also explore preventive surgeries or chemoprevention medications.
5. Talk to Your Family
Open conversations can save lives. Munn’s insistence that her mother and sister take the risk assessment directly led to her mom’s early diagnosis. Knowledge really is power when it comes to hereditary cancer risk.
Olivia Munn’s openness about her breast cancer journey and now her mother’s—shines a light on the power of preventive care and family advocacy. Her message is clear: Don’t wait for symptoms. Know your risk. Act early.
“Anything above 20% is considered high risk, and you should insist your doctor order a breast MRI,” she urged in her Instagram post.
And while genetics may predispose us to certain conditions, timely testing, awareness, and action can change the outcome. In this case, one family’s proactive approach saved two lives. It could do the same for yours.
© 2024 Bennett, Coleman & Company Limited

