- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Does Chemotherapy Cause Dementia?

Credit: Canva
Chemotherapy is a widely used cancer treatment involving powerful medications that target and destroy cancer cells. However, these drugs can also affect healthy cells, leading to various side effects. According to the American Cancer Society, common side effects of chemotherapy include fatigue, hair loss, infection, easy bruising or bleeding, anaemia, mood changes, and cognitive difficulties often referred to as 'chemo brain.'
The relationship between chemotherapy and dementia remains a subject of debate among researchers. Conflicting studies present differing perspectives on the potential link. For instance, a 2017 study examined the risk of Alzheimer’s disease in female breast cancer survivors. The researchers noted that those who underwent chemotherapy might be at an increased risk of Alzheimer’s, particularly if they experienced changes in specific brain structures. However, they also observed that women who did not receive chemotherapy also had a higher risk, suggesting that structural changes in the brain might contribute to Alzheimer’s rather than the cancer treatment itself.
Conversely, a 2021 study involving 135,834 individuals aged 65 and older with colorectal cancer indicated that chemotherapy might actually reduce the risk of Alzheimer’s and other forms of dementia. Further complicating the narrative, a 2022 longitudinal study found no definitive evidence linking chemotherapy to an increased risk of dementia. These varying outcomes underscore the need for more comprehensive research to better understand the connection between chemotherapy and cognitive decline.
While there is no cure for dementia, several treatment strategies can help manage its symptoms. Medications such as cholinesterase inhibitors and NMDA receptor antagonists are commonly prescribed to slow cognitive decline. Additionally, drugs to manage blood pressure, cholesterol, and depression can also be beneficial. Non-pharmaceutical approaches, such as regular physical activity, a balanced diet, avoiding alcohol and smoking, and staying socially active, may also help mitigate symptoms and improve overall brain health.
Cheotherapy Also Causes Other Neurological Conditions
Beyond the potential link to dementia, chemotherapy can cause other neurological effects. 'Chemo brain,' characterised by memory problems, difficulty concentrating, and trouble multitasking, is one of the most reported cognitive side effects. While these symptoms are typically temporary, they can persist in some individuals. Furthermore, chemotherapy can lead to neurotoxicity, which may result in limb weakness, numbness, headaches, and cognitive or behavioural issues.
Given the conflicting evidence on chemotherapy’s long-term effects on the brain, further studies are essential to establish a clearer understanding of the risks. In the meantime, patients undergoing chemotherapy should discuss any cognitive concerns with their healthcare providers and explore ways to manage potential side effects effectively.
UK Heatwave Warning: This Common Fan Mistake Could Raise Your Heart Attack Risk, Study Warns

Credits: Canva
August’s scorching weather has made even short trips outside exhausting and turned nights into sweaty, sleepless ordeals. With no air conditioning in many homes, people are using ice packs, wet towels, frozen water bottles and electric fans to cope. But a fan might not be doing you any favours; in fact, if you use it the wrong way, it could be raising your risk of a heart attack.
What the study says
A new study from the University of Sydney has found that while fans can make you feel blissful during a heatwave, they can also push your body into dangerous territory, particularly if you’re dehydrated. Researchers wanted to understand exactly how fans affect our bodies in hot and humid conditions, so they put 20 volunteers into a climate-controlled space set to 39.2 degrees Celsius with 49 per cent humidity.
The participants weren’t just asked to sit there and suffer. Scientists tracked their heart rate, core temperature, sweating, and comfort levels, both when they were properly hydrated and when they had been deliberately dehydrated (by avoiding fluids and water-rich foods for 24 hours). Each hydration state was tested twice — once with a fan blowing and once without.
Sweat, strain, and a shocking finding
The study revealed that using a fan while dehydrated increased sweat losses by around 60 per cent. Now, sweating might sound like a good thing when you’re overheating, but in this case, it pushed the body into a dangerous loop: more sweat loss means more dehydration, which means more cardiovascular strain. That extra strain can, in extreme cases, trigger heart attacks, particularly in vulnerable individuals.
Connor Graham, PhD, who led the study, explained that fans can help keep you cooler at temperatures up to around 39–40 degrees Celsius. But when the air gets hotter than your skin, the fan can actually heat your body faster than it can cool itself. Most extreme heat decedents do not have air conditioning but often own electric fans, he said. In hotter conditions, fans should be turned off, as they can worsen heat stress.
Why hydration is important
The study says that hydration is a game-changer. When the volunteers were well hydrated, fans were far less risky, even in the extreme heat chamber. But when hydration levels were low, the fan’s effects tipped from helpful to harmful, sending heart rate and body strain higher.
This is because sweat is our body’s primary cooling mechanism. When you’re hydrated, you can produce enough sweat to evaporate and take heat away from your body. But if you’re already running on empty, a fan simply accelerates fluid loss without actually cooling you enough. That’s like trying to run your car on fumes while flooring the accelerator.
Heatwaves, Britain, and fan habits
In the UK, people are not exactly built for this kind of weather. Their homes are designed to trap warmth, not keep it out, which is why fans are practically flying off the shelves in high street stores right now. But unlike in countries where air conditioning is the norm, we often rely on fans as our only cooling option and that’s where this warning matters.
It’s easy to assume that “more fan” means “more cool”. The reality is a bit more complicated. In moderately hot weather, a fan can help your sweat evaporate and keep you feeling comfortable. In extreme heat, particularly if your flat feels like a slow cooker, a fan can just push hot air onto your body, speeding up dehydration and heart strain.
How to use fans safely in a heatwave
Don’t chuck your fan in the bin just yet. Here are some science-backed ways to stay safe while keeping cool:
- Stay hydrated first — Drink water regularly throughout the day, not just when you feel thirsty. Herbal teas and diluted juices also count.
- Use fans strategically — Aim them across your body, not directly at your face, and combine them with open windows to improve airflow.
- Cool the room, not just yourself — Place a bowl of ice in front of the fan so it blows chilled air.
- Turn it off during extreme heat — If indoor temperatures are hotter than 40 degrees Celsius (or feel like it), skip the fan and switch to other cooling methods.
- Know your limits — Older adults, people with heart conditions, and those on certain medications are more vulnerable to heat strain.
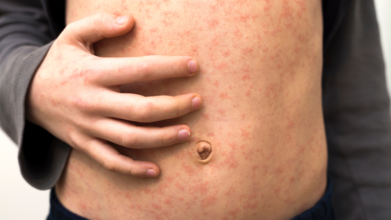
Ottawa Measles Update: Ottawa Public Health Warns Residents Against Measles Amid Its 5th Case
Credits: Canva
Ottawa Public Health (OPH) is urging residents to monitor for symptoms of measles after confirming the city’s fifth case of the year. Officials say the individual, who has not been identified, likely contracted the virus while travelling in western Canada. While the risk to the general public remains low, OPH warns that certain people may have been exposed at specific locations around the city between August 5 and 8.
Potential Exposure Sites in Ottawa
In its Thursday alert, OPH listed several businesses and venues where possible exposure could have occurred:
- Shoppers Drug Mart, 702 Bank St., Aug. 5 from 9 p.m. to 11:30 p.m.
- Michaels, 165 Trainyards Dr., Aug. 5 from 8:15 p.m. to 10:30 p.m.
- Fitness Lab, 34 Beech St., Aug. 6 from 5:45 a.m. to 8:45 a.m.
- Wilf and Ada’s, 510 Bank St., Aug. 7 from 12:30 p.m. to 4 p.m.
- Izakaya Shingen, 201 Bank St., Aug. 8 from 5 p.m. to 8 p.m. Cineplex Odeon, 2385 City Park Dr., Aug. 8 during the 6:50 p.m. screening of Fantastic Four: First Steps
Officials advise anyone who visited these locations during the listed times to watch for symptoms such as cough, fever, red eyes, and rash for 21 days after the possible exposure.
Exposure at Ottawa Hospital’s Emergency Department
The Ottawa Hospital has confirmed the same patient visited the General campus emergency department on August 11, waiting for several hours before being assessed in the early hours of August 12. Dr. Eric Eckbo, an infection control physician at the hospital, said measles was suspected during the examination. Infection control measures, including masking and isolation, were immediately implemented, and OPH was contacted.
Hospital staff are now following up with anyone who may have been exposed during that time, including immune-compromised individuals at higher risk of complications. Most people exposed will not develop measles due to immunity from vaccination or previous infection.
ALSO READ: Measles Death In Liverpool Highlights Vaccine Urgency For Children: Here's What Parents Need To know
Part of a Larger Outbreak
Ontario has been dealing with a significant measles outbreak this year, with 2,362 cases reported as of August 12, according to Public Health Ontario. Two of Ottawa’s five cases are linked to this provincial outbreak. Alberta is also experiencing a large number of cases, with dozens of new infections reported weekly.
No Local Transmission Detected
Despite these numbers, OPH says there is still no evidence of local measles transmission in Ottawa in 2025. Health officials credit this to high vaccination coverage in the community. However, they stress that measles remains one of the most contagious viruses and that the measles-containing vaccine is the most effective protection.
Advice for the Public
People who develop symptoms are urged to contact their primary healthcare provider before visiting any medical facility. If a hospital visit is necessary, they should wear a mask and inform staff immediately upon arrival. Those unable to get vaccinated, such as infants and people with compromised immune systems, are particularly vulnerable and should take extra precautions.
Canada eliminated measles in the 1990s due to strong vaccination programs, but declining immunization rates worldwide are fueling its return. Ottawa health officials are reminding residents that staying up to date on vaccinations remains the best way to prevent outbreaks.
Mounjaro Price Hike: Here's All That You Need To Know About This Weightloss Drug

Credits: Canva
From September, Eli Lilly will raise the UK price of its diabetes and weight-loss drug Mounjaro by as much as 170%. The US pharmaceutical giant says the increase will align UK costs with those in other developed nations and address “pricing disparities.”
The NHS will not be affected for now. The price surge is aimed at private patients and providers, who often negotiate discounts behind closed clinic doors. But for those paying out of pocket, the jump is steep, the highest monthly dose will soar from £122 to £330, while lower doses will rise by 45 to 138 per cent.
For many, this is more than a wallet shock. It could mean rethinking whether to continue treatment, especially since Mounjaro is often taken long term to maintain results. With so much at stake, here’s a closer look at what the drug does, who it’s for, and the benefits and risks to consider.
What is Mounjaro?
Mounjaro, the brand name for tirzepatide, is an injectable medication, notes Diabetes UK, and is approved in the UK for type 2 diabetes and, more recently, for obesity. It is part of a newer class of drugs that not only control blood sugar but also promote significant weight loss.
Unlike earlier medications such as Ozempic and Wegovy, both of which were based on semaglutide, Mounjaro works by activating two hormone receptors: GLP-1 and GIP, at the same time. This “dual agonist” approach appears to produce greater weight loss than single-receptor drugs.
How Does it Work?
Mounjaro increases levels of natural hormones called incretins. These hormones help the body release more insulin when needed, reduce glucose production by the liver, and slow digestion so you feel fuller for longer.
The result is a two-pronged effect:
Better blood sugar control in people with type 2 diabetes
Reduced appetite and calorie intake leading to weight loss
In clinical trials, people taking the highest dose (15 mg weekly) lost up to 21 per cent of their body weight. That’s on par with some bariatric surgeries, but without the invasive procedure.
Who Can Take Mounjaro?
For type 2 diabetes
Adults aged 18 and over who have not been able to control blood sugar with other medications, or who cannot tolerate them due to side effects or other conditions.
Typically prescribed if the person also has a BMI of 35 kg/m² or higher with obesity-related health issues, though exceptions exist for those with lower BMIs in certain ethnic groups or specific medical needs.
For obesity
In England and Wales: Recommended for people with a BMI of at least 35 kg/m² and related health conditions, including type 2 diabetes. Lower thresholds apply for some ethnic groups.
In Scotland: Available for people with a BMI of at least 30 kg/m² plus one obesity-related condition.
The Benefits
Significant weight loss that can improve or reverse obesity-related health problems
Improved blood sugar control in people with type 2 diabetes
Once-weekly dosing with a pre-filled pen for convenience
May reduce risk of complications from diabetes, though more research is ongoing for cardiovascular benefits
The Risks and Side Effects
Like other drugs in its class, Mounjaro can cause:
- Nausea, vomiting, diarrhoea, constipation, indigestion
- Risk of low blood sugar if taken with insulin or certain other diabetes drugs
- Possible risk of high blood sugar if insulin doses are cut too quickly
- In animal studies, an increased risk of thyroid tumors, whether this applies to humans is still unknown
Long-term safety data is limited since the drug is relatively new. Some people may also regain weight if they stop taking it.
Cost and Access Challenges
On the NHS, Mounjaro is free for those eligible under treatment guidelines, but rollout is gradual due to costs and support service limitations. Access for weight loss alone is prioritized for those with the highest clinical need.
Private prescriptions vary in cost and availability. After the September price hike, the financial burden will be significant for many patients, especially since ongoing treatment is often required to maintain benefits.
Life Without the Jab
If the higher cost puts Mounjaro out of reach, lifestyle changes can still deliver meaningful results. Strategies that mimic some of its effects include:
- Eating high-protein meals to promote satiety
- Choosing high-fibre foods to slow digestion
- Limiting ultra-processed foods that spike blood sugar
- Regular physical activity to improve insulin sensitivity
© 2024 Bennett, Coleman & Company Limited

