- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Eli Lilly’s Mounjaro Kwikpen Gets Indian Approval For Diabetes And Weight Loss
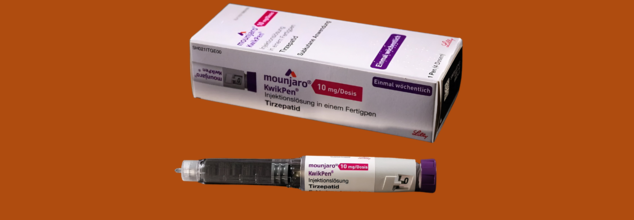
Eli Lilly announced that its breakthrough drug, Mounjaro (tirzepatide), has received marketing authorization from India’s apex drug regulator, the Central Drugs Standard Control Organisation (CDSCO). The approval is for the KwikPen presentation specifically— a prefilled multi-dose injection pen to be used once a week.
This milestone puts India in step with an increasing number of nations that are adopting next-generation therapies for the control of chronic metabolic diseases. Yet beyond the regulatory headlines, this approval has profound implications for public health, clinical practice, and the pharmaceutical industry.
Mounjaro KwikPen is a new prescription drug that is intended to provide both diabetes and weight management care in one easy-to-use delivery form. The KwikPen is an on-body, single-patient-use, prefilled pen that delivers four fixed doses of 0.6 mL in six strengths—2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, and 15 mg. It is taken once weekly, both user-friendly and patient-compliant compared to previous daily injectable therapies.
What sets Mounjaro apart from other GLP-1 drugs is its unique dual-action mechanism: it is the first and only drug that does this at the same time, engaging both GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) receptors. Both are naturally occurring incretin hormones that affect blood sugar and hunger.
These complex actions not only treat type 2 diabetes but also offer significant weight loss advantages, especially in those with a BMI of 30 or more, or in those with a BMI of 27 or greater who have a comorbidity related to weight. India, on the other hand, is facing a double public health challenge: explosively rising diabetes and obesity rates. It is estimated that more than 101 million Indians are living with diabetes today, with a large number of those facing challenges in having optimal glycaemic control. Adult obesity prevalence was at 6.5% in 2023 and affected almost 100 million individuals. In the NFHS-5 (2019–21) survey, 24% of females and 23% of males aged 15–49 were overweight or obese—presenting a dramatic increase compared to earlier years.
Projections from the International Diabetes Federation indicate that the number of adults with diabetes in India could soar to 124 million by 2045. In light of these alarming statistics, the introduction of an effective, once-weekly injectable therapy like Mounjaro, which addresses both blood sugar control and weight management, could prove to be a transformative breakthrough for millions across the country. The approval of Mounjaro KwikPen represents an advancement in the treatment of individuals with type 2 diabetes and obesity, providing an easy means for patients to administer their medication," said Winselow Tucker, President and General Manager, Lilly India. He pointed out the convenience of six strength options, adding that individualized therapy is an essential component of effective chronic disease management.".
Healthcare professionals will embrace the potential to titrate dose according to individual patient goals, whether glycemic, weight loss, or both. Ease of use for KwikPen also simplifies the burden on patients, enhancing adherence in the long term.
How Does Mounjaro Work in the Body?
The physiology behind Mounjaro is both intriguing and revolutionary. Tirzepatide, the active compound in Mounjaro, binds with selectivity to both GIP and GLP-1 receptors and initiates a cascade of metabolic effects that include:
- Increased insulin secretion in response to increasing glucose levels.
- Inhibition of glucagon, a hormone that increases blood glucose.
- Increased insulin sensitivity, enabling the body to process glucose more efficiently.
- Slowed gastric emptying, resulting in increased satiety and decreased food consumption.
- Appetite control through central nervous system mechanisms, particularly in brain areas responsible for hunger signaling.
- Decreased fat mass and enhanced lipid metabolism, with a role in weight loss.
Mounjaro's approval in India follows closely on the heels of Novo Nordisk's worldwide launch of Wegovy, a semaglutide-based weight loss injection. Although Wegovy is not yet available in India, there is anticipation. Local players in the pharma sector are not waiting, though.
As semaglutide patents in India are set to expire by 2026, indigenous players are already scrambling to develop cheap versions of both Wegovy and Mounjaro. This has the potential to drastically bring down prices and increase access over the next few years.
That being said, Lilly has not yet announced Mounjaro's launch date or official price in India, although company sources point to an imminent announcement.
Mounjaro has already caused ripples across the world—achieving FDA approval in the United States, and seeing rampant adoption in patients with type 2 diabetes and obesity. Its launch in India is not merely a business strategy—it is a public health necessity.
Mounjaro's inclusion in India's healthcare system could fundamentally change how doctors address interrelated conditions such as insulin resistance, metabolic syndrome, and cardiovascular risk facilitated by obesity.
Oxford Researchers Develop 'One-Dose' Malaria Vaccine; Could It Transform Global Prevention Efforts?
Malaria is still one of the most intractable and deadly diseases, Despite decades of effort, the disease continues to claim nearly 600,000 people annually, with most of the victims being children under the age of five in the poorest parts of the world. Although the advent of vaccines like RTS,S (Mosquirix) and R21 has been an important milestone, the problem of administering multiple doses in resource-poor environments has slowed the world's efforts against malaria. A groundbreaking team at the University of Oxford has now pioneered a game-changing solution: a single-dose malaria vaccine based on microcapsule technology, which has the potential to revolutionize the way we engage with immunization in the world's most vulnerable populations.
In 2023 alone, there were reported cases of 263 million malaria cases globally, and this clearly demonstrates the need to have more efficient prevention methods. The World Health Organization (WHO) has proposed an ambitious goal—90% protection against Plasmodium falciparum (Pf) infection—but present vaccines are not up to the task. Mosquirix, the first WHO-approved malaria vaccine, needs a minimum of three injections and provides only 39% efficacy. R21, created by Oxford and licensed in 2023, enhanced efficacy to 77% with the assistance of Novavax's Matrix-M adjuvant but continues to require two to three doses for maximum protection.
These multi-dose regimens pose a strong obstacle in areas where the healthcare infrastructure is thin. Booster visits are missed, making millions—particularly children—susceptible to infection. Logistical and economic costs of repeated clinic visits, cold chain storage and distribution add to the obstacles to making widespread immunisation.
Published in Science Translational Medicine, the results come at a critical juncture. Malaria remains a devastating epidemic, with more than 263 million cases reported worldwide and close to 600,000 reported deaths in 2023, many of which were in children under five years old. As global health leaders increasingly search for more impactful, scalable solutions, this one-shot vaccine is a bold move toward the World Health Organization's (WHO) ambitious target of 90% protection against Plasmodium falciparum infections.
Despite decades of study and billions spent, malaria is still endemic in more than 90 countries, overwhelmingly impacting the world's poorest regions. One of the longest-standing challenges in combating malaria has been the challenge of keeping vaccination schedules—a particular issue in isolated communities with limited clinic access or refrigeration.
The Oxford microcapsule platform, led by Professor Romain Guyon's team, the new vaccine formulation encloses the malaria antigen in microscopic, biodegradable spheres of PLGA (poly-lactic-co-glycolic acid). The vaccine is administered once, and the microcapsules release a priming dose instantaneously, a subsequent booster dose delivered time-released inside the body, simulating the conventional multi-dose regimen.
"Our microcapsules function like controlled-release vaults," says Guyon. "They deliver the booster on a pre-programmed schedule—two weeks to several months—so the immune system sees a second dose without needing the patient back into the clinic."
This novel timed-release is enabled by chip-based microfluidics technology, enabling researchers to have fine control over capsule size, degradation rate, and release time. Most importantly, the platform can be integrated with standard pharmaceutical manufacturing processes, meaning rapid scaling is both viable and affordable.
In animal models, the one-dose vaccine had 85% efficacy, comparable to that provided by current two-dose regimens. Antibody levels were strong for as long as 11 weeks, and immune response was strong even after refrigeration at 4°C for 4–7 weeks, validating the stability of the vaccine in refrigerated storage conditions.
This holds especially great promise for low-resource environments, where the cold chain—the requirement to keep vaccines at particular temperatures—tends to fail. The microcapsules, at 65 micrometers in diameter, are injectable through regular syringes, with no specialized delivery apparatus.
Dr. Luca Bau of Oxford’s Institute of Biomedical Engineering emphasizes the impact, “Reducing the number of clinic visits could make a major difference in communities where healthcare access is limited. Our goal is to eliminate the structural barriers that prevent people from accessing life-saving innovations.”
As per WHO, 20.5 million children fell behind on routine vaccinations in 2022 alone, many because of logistical challenges a single-dose vaccine would address. Furthermore, malaria disproportionately kills children, and of those deaths, most occur among children younger than age five in sub-Saharan Africa.
Conventional malaria prevention methods—such as insecticide-treated bed nets, anti-malarial drugs, and indoor spraying—have also advanced, but drug resistance and vector adaptation undermine their effectiveness. Vaccines are still the most scalable long-term option, and a single-dose formulation would have the potential to make uptake and outcomes far better.
Although the target now is malaria, scientists indicate the microcapsule technology may be adapted to suit many vaccines and therapies, especially those needing repetitive dosing or complicated regimens. Such vaccines include those for tuberculosis, HPV, as well as future pandemic countermeasures.
Dr. Guyon expounds, "Our technology solves three fundamental problems—programmability, injectability, and scalability. If safe and effective in humans, it has the potential to transform how we administer everything from pediatric vaccines to cancer immunotherapies."
The team is currently gearing up for human clinical trials, the second most important step toward establishing safety and efficacy in diverse populations.
Commonwealth Swimmer Archie Goodburn And His Journey With A Rare Cancer That Kills Before 40

(Credit-Archiegoodburn/Instagram)
“It’s a moment I’ll never forget, when I sat down at the edge of the pool – the pool where I’ve trained my whole life – to find out there’s a brain tumor”
In 2024, Archie Goodburn, a- young and promising 23-year-old athlete (currently 24-year-old) who won a bronze medal at a major junior championship and swam for Scotland, shared difficult news of being diagnosed with a rare form of cancer. This Scottish swimmer who has a record in the 50 m breaststroke found himself in a position where he might have to put a stopper on his dreams of competing in the Paris Olympic. He was diagnosed with incurable brain tumors. This discovery came after he experienced numbness and seizures, especially around the time he was trying to qualify for the Olympics.
Also Read: World Drug Day 2025: Why Is It Important For Us To Observe This Day?
Incurable Brain Cancer And Impending Time Limit
Tests after the Olympic trials showed Archie has three large brain tumors called oligodendrogliomas that can't be removed with surgery. These tumors are rare and usually grow slowly, forming from a type of brain cell. While they're more often found in adults, they can affect anyone. Archie will now be undergoing radiotherapy and chemotherapy to treat them.
Also Read: Hundreds Report Pancreatitis After Using Weight-Loss Drugs; UK Launches A Study To Investigate
The brain tumor is in a critical area and is the leading cancer killer for people under 40, according to Brain Tumour Research.
Taking to social media Archie explained that he started having strange episodes of dizziness and was feeling uncomfortable in late 2023 that messed up his training. At first, they thought these were severe migraines. They caused him to lose strength, feel numb on his left side, and experience fear, nausea, and strong déjà vu. He now knows these were actually seizures.
Also Read: Hundreds Report Pancreatitis After Using Weight-Loss Drugs; UK Launches A Study To Investigate
What Is Oligodendrogliomas?
According to the US National Cancer Institute, Oligodendroglioma is a type of tumor that starts in the brain or spinal cord. To correctly diagnose it, doctors look for two specific changes in the tumor's genes: a specific gene change called an IDH mutation and a particular loss of parts of chromosomes 1 and 19.
Archie on his Instagram account said, “My tumors express a mutation of my IDH1 gene that is shared with some forms of leukaemia.” According to a 2012 study published in the Brain Pathology, IDH1 gene is a mutation that happens in the early stages of brain development and is a common step in the development of brain tumors. This type of gene mutation is common in glial tumors, which are a type of tumor that originated in the glial cells present in the brain and spinal cord.
To get a clear diagnosis, doctors usually need to take a small piece of the tumor during surgery, if possible. A neuropathologist then looks at this piece of the tumor.
The National Cancer Institute explains that the causes of this brain tumor are still unknown. However, being exposed to radiation and having certain gene changes passed down in families might increase the chance of getting them.
What Are The Chances of Survival for Oligodendrogliomas?
Prognosis is about the likely outcome of the disease or the chance of recovery. It depends on several things: Type, grade, location, spread and severity of the type of tumor, specific gene changes, the patient's age, and how much of the tumor is left after surgery (if surgery was possible). Archie said, “IDH inhibiting medications, a new class of drug, have seen phenomenal developments in the last few years with some pretty amazing results. Continued progress in this field will aid my prognosis massively.”
About 79.5% of people with oligodendroglioma are still alive five years after diagnosis. However, one must remember that there are many things that affect this number. These include the tumor's grade and genetic makeup, the person's age and health when diagnosed, and how well they respond to treatment. If you want to understand your own outlook, it's best to talk to your doctor.
Archie's Positive Outlook
Despite the tough news, Archie is staying incredibly positive. He mentioned a "silver lining": these types of tumors often respond better to radiotherapy and chemotherapy than some other serious brain tumors. He also noted they usually grow slowly and might have been there for years.
Archie is determined to face this challenge head-on. He's drawing strength from being young and fit, and from the amazing support he gets from his friends, family, and girlfriend. He plans to "remain positive and to keep being Archie." Many people have applauded his strength and resilience to bring awareness about the disease “I'm lucky... I've got time to shout about this disease.”
FDA Updates COVID Vaccine Labels Over Rare Heart Inflammation Risk: What You Need to Know?
Credits: Canva
The U.S. Food and Drug Administration (FDA) has updated its warning labels for the two most widely used COVID-19 vaccines — Pfizer and Moderna — to provide more detailed information about the rare risk of myocarditis, an inflammation of the heart muscle. The condition has primarily been observed in younger males and was first identified shortly after the vaccines became widely available in 2021.
FDA Demands More Detailed Labeling
Though both vaccine makers had already included myocarditis warnings in their prescribing information, the FDA formally asked them in April to expand these details. The agency can mandate label changes, but such updates are typically negotiated with drugmakers.
The revised warning now estimates that 8 cases of myocarditis occur per 1 million people who received the 2023–2024 COVID vaccines in the age group of 6 months to 64 years. The update also clarifies that the risk is highest among males aged 12 to 24, a slight expansion from the earlier label, which focused on boys aged 12 to 17.
Conflicting Safety Findings at Top U.S. Agencies
Interestingly, this update appears to contradict some earlier findings from the Centers for Disease Control and Prevention (CDC). The CDC had previously stated that, based on vaccine safety data through 2022, there was no significant increase in myocarditis risk linked to COVID-19 vaccines. They also pointed out that vaccine-related myocarditis cases tend to be mild and resolve quickly, especially when compared to heart inflammation caused by the COVID-19 infection itself.
How This New Expert Panel Will Strengthen Vaccine Oversight
The timing of the FDA’s label change aligns with broader shifts in vaccine policy under the leadership of Health Secretary Robert F. Kennedy Jr. A longtime vaccine skeptic, Kennedy recently dismissed all 17 members of the CDC's vaccine advisory panel and appointed a new group, some of whom have expressed anti-vaccine sentiments in the past. This week marked their first meeting, where ongoing use of COVID-19 vaccines, especially among vulnerable groups like pregnant women, was discussed.
Also Read: Can Airborne Fungi Predict COVID and Flu Outbreaks? New Research Says Yes
Covid Vaccine: New Restrictions and Requirements Under Review
FDA Commissioner Dr. Marty Makary, appointed by Kennedy, has taken several steps to limit the scope of COVID-19 vaccine recommendations. Recently, he restricted annual COVID-19 booster shots to seniors and high-risk individuals. Makary has also suggested that seasonal updates to vaccines — meant to better match circulating virus strains — should be treated as entirely new products requiring additional clinical testing.
Focus Should Be on Understanding Risk, Not Curtailing Vaccines
While acknowledging that myocarditis should not be dismissed, some public health experts believe the FDA’s latest approach is flawed. “We should be investigating who is prone to myocarditis to see if we can predict and mitigate that risk,” said Dr. Robert Morris, a public health specialist at the University of Washington. “They are right to raise the concern, but not with these broad warning changes," as reported by Associated Press.
Makary and his team had previously questioned the government’s booster recommendations. In a 2022 paper, he and two FDA colleagues argued that mandating boosters for young people might lead to more harm than good, a position that diverged from CDC guidance at the time.
© 2024 Bennett, Coleman & Company Limited

