- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
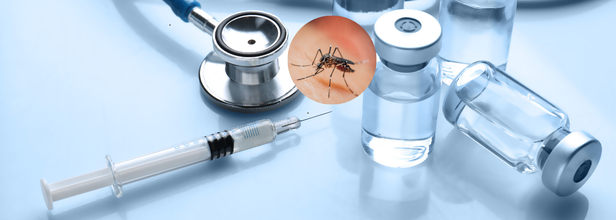
India’s First Dengue Vaccine Nears Final Trial Stage
Credits: Canva
In a significant stride toward curbing the spread of vector-borne diseases, India is moving closer to having its first indigenous dengue vaccine. The Indian Council of Medical Research (ICMR), in collaboration with Panacea Biotec, is on track to complete the enrollment of participants for Phase 3 clinical trials by October 2025, according to CNBC-TV18.
This crucial phase of testing, launched on August 14, 2024, marks the most advanced stage yet for the vaccine’s development. The multi-center, double-blind, randomised, placebo-controlled study is designed to assess the vaccine’s efficacy, safety, and long-term immunogenicity. So far, over 70% of the targeted 10,335 participants have been enrolled across 20 medical centers nationwide, according to CNBC-TV18 sources.
Two-Year Follow-Up Required Before Regulatory Submission
Once participant enrollment is completed, each subject will be monitored for a two-year follow-up period. This extended observation window, considered critical for evaluating the vaccine's long-term safety and effectiveness, is expected to conclude by the end of 2027.
According to CNBC-TV18, sources familiar with the development confirmed, “The enrollment process is progressing smoothly, and no safety concerns have been reported so far.”
Only after the follow-up period ends and the data are thoroughly analyzed will the findings be submitted to the Central Drugs Standard Control Organization (CDSCO) for regulatory review and potential market authorization. “If the vaccine demonstrates acceptable efficacy and a clean safety profile, it could then be considered for regulatory approval and subsequent launch,” CNBC-TV18 reported, quoting official sources.
ALSO READ: Dengue On The Rise: How Climate And Geography Are Shaping A Growing Threat
National-Scale Trial Across Premier Institutions
The Phase 3 trial is being carried out at reputed medical institutions located in major cities including Chennai, Pune, Hyderabad, Bengaluru, New Delhi, and Kolkata. According to CNBC-TV18, the study protocol stipulates a comprehensive two-year post-vaccination follow-up. The final data from this period will be crucial in determining the vaccine’s fate in the Indian market.
A Single-Dose Vaccine: What Is It All About?
What sets this vaccine apart from global counterparts is its single-dose formulation. This is expected to ease the logistical and financial challenges of mass immunization in dengue-endemic areas. Unlike international vaccines like Sanofi’s Dengvaxia and Takeda’s QDENGA—which require multiple doses and have limitations in efficacy across different dengue virus serotypes—India’s homegrown candidate offers a potentially more accessible and broadly applicable alternative.
Earlier phases of clinical evaluation, including Phase 1 and Phase 2 trials conducted in India, had already received regulatory clearance from CDSCO. These trials showed that the vaccine was both safe and capable of eliciting a strong immune response.
“India’s first dengue vaccine candidate has shown encouraging results so far. Phase 1 and 2 trials confirmed that the vaccine is safe and induces a protective immune response. We’re hopeful that Phase 3 will bring us closer to a much-needed tool in dengue prevention,” CNBC-TV18 reported, citing official sources.
YOU MAY LIKE: India’s First Indigenous Dengue Vaccine Coming Soon; What To Expect?
A Vital Step for a Country with High Dengue Burden
India continues to suffer from seasonal dengue outbreaks, with thousands of hospitalizations each year placing strain on public health infrastructure. The World Health Organization estimates about 390 million dengue infections occur globally each year, with India accounting for a significant portion. With no specific antiviral treatment available, prevention through vaccination and vector control remains the most effective strategy.
As per current projections, enrollment will wrap up by October 2025, followed by a two-year follow-up until the end of 2027. Only after this can ICMR and Panacea Biotec submit their formal application for market authorization, CNBC-TV18 noted.
If the vaccine passes regulatory scrutiny, it could revolutionize dengue control efforts not only in India but also in other low- and middle-income countries grappling with high disease burdens and limited access to effective vaccines.
‘Kissing Bug’ Epidemic In US: What You Need To Know About Chagas Disease, Is it Contagious?

(Credit-UCLA Canva)
US Health officials have declared Chagas disease, a potentially deadly infection spread by the ‘kissing bugs’, an epidemic. These bugs have been found in 32 states, and at least eight Americans have caught the disease from local transmission. This has led scientists to urge the CDC and the WHO to officially declare the illness as endemic in the U.S., which means it's a disease that is naturally present in the region. Experts believe that over 300,000 people in the U.S. may have Chagas disease, but very few of them know it.
What Is Chagas Disease?
According to UCLA Health, Chagas disease, a potentially life-threatening condition, is most commonly spread by the “kissing bug,” which gets its name because it tends to bite people on the face while they sleep. After biting, the bug defecates on the skin, leaving behind a parasite called T. cruzi. When the person scratches the itchy bite, they accidentally rub the parasite into the wound, and that's how it enters the bloodstream. Chagas disease can also be passed from a pregnant person to their baby, through organ transplants and blood transfusions, or by eating uncooked food that has been contaminated with the parasite.
Is Chagas Disease Contagious?
No, the Cleveland Clinic explains that this disease cannot spread from person to person. Many people with Chagas disease have no symptoms at all, which is why it's often called a "silent disease." In the early stage, which lasts about two months, some people might experience a swollen eyelid, fever, or body aches, but these symptoms are easily mistaken for other common illnesses.
Over a person’s lifetime, about 20% of those infected will develop serious, long-term problems, most often affecting the heart. Chagas disease can slowly damage the heart, leading to an enlarged heart, heart failure, or even a heart attack. It can also cause digestive problems, like an enlarged colon.
What Are Signs And Symptoms of Chagas Disease?
According to the World Health Organization (WHO), Chagas disease happens in two main stages. For most people, the first stage goes unnoticed, but it can lead to very serious problems later on.
The First Stage
This early phase lasts for about two months after a person is infected. Most people have no symptoms at all, or they experience mild, general symptoms that could be caused by anything else, such as a fever, headache, muscle pain, or swelling. In rare cases, a person might see a specific sign, like a skin sore where they were bitten, or a purple swelling of one eyelid.
The Second Stage
The second stage of the disease can last for a person's entire life. During this time, the parasites hide mainly in the heart and muscles of the digestive system. About 10 to 30 years after being infected, up to a third of people will develop serious heart problems, and up to 1 in 10 will have digestive issues, such as an enlarged food pipe or colon. Over time, this can lead to an irregular heartbeat, slow heart failure, and in some cases, sudden death.
How To Treat Chagas Disease?
Anti-parasite medications are only effective at treating Chagas disease during its very early stage. Once the disease has been in the body for a while, there is no cure, though symptoms of heart failure can be managed with medication or even a heart transplant.
Doctors recommend that anyone who is from or has traveled to Latin America and begins to experience heart problems should be tested for Chagas disease. It's also worth noting that the U.S. blood supply has been tested for Chagas since 2007.
How Can We Prevent Chagas Disease?
WHO explains that because Chagas disease is carried by "kissing bugs" and is so widespread among wild animals throughout the Americas, it's not possible to completely wipe it out. Instead, public health efforts are focused on stopping the disease from spreading to humans, making sure infected people get diagnosed early, and providing them with lifelong medical care. To prevent the spread of Chagas disease, health organizations recommend a few key approaches:
Controlling the bugs
This is considered the most effective way to prevent the disease. It includes spraying homes and surrounding areas with bug-killing chemicals and making sure houses are built and kept clean to prevent the insects from living in cracks in the walls.
Personal protection
People can protect themselves with personal measures like using bed nets and practicing good hygiene when handling food.
Screening donors
Screening blood donors is necessary to prevent the disease from spreading through blood transfusions and organ transplants.
Checking newborns
It is also important to test newborns and other children of infected mothers, as the disease can be passed during pregnancy.
NHS Says Martha’s Rule To be Followed At All Hospitals In England: All About This New Rule?

(Credit-Merope Milis)
Martha's rule is a scheme rolled out in 2021 after the death of a 13-year-old, NHS has now declared that all acute inpatient sites will offer the service, that has now saved thousands of lives.
Martha’s rule was set up after the death of young Martha Mills who developed sepsis while she was under the care of King’s College Hospital under NHS Foundation Trust in south London in 2021.
Her death was a wakeup call for health authorities and her parents, both of whom had brought up their concerns regarding their daughter’s care multiple times, while they were completely ignored. Having this facility could help many families feel at ease and know that they can easily ask for second opinions.
Who Is Martha from Martha's Rule?
What if you knew your loved one was getting sicker in the hospital, but no one was listening? That's the heartbreaking situation that led to Martha's Rule, a new system designed to make sure every patient's voice is heard.
In 2021, Martha was in the hospital for an injury. While she was there, she developed a serious and life-threatening condition called sepsis. Her family saw that she was getting sicker and sicker, and they frantically tried to get the doctors and nurses to listen to their fears. Sadly, they were not heard, and Martha passed away.
The rule was put in place when the authorities ruled out that young Martha may have survived if the doctors had identified the warning signs of her declining condition and transferred her into intensive care. Her parents, in their grief, decided to fight to make sure no other family would have to go through the same tragedy.
How Does Martha's Rule Work?
Martha's Rule is a way to make sure that patients, families, and caregivers can have their concerns heard and addressed in a hospital. It is being introduced for patients staying overnight in hospitals across England. The rule has three main parts:
- Patients will be asked daily how they are feeling, and their answers will be officially recorded.
- Hospital staff can, at any time, ask for an urgent review from a different team if they are worried about a patient but feel their concerns are not being listened to.
- Patients, their families, and caregivers will always have a way to ask for this urgent review as well. This will be clearly advertised in the hospital.
If a family member or patient feels worried about a worsening condition and their concerns are not being addressed by their main care team, they can call a dedicated number for a rapid review from a different, independent team.
Has Martha’s Rule Saved Anyone?
According to NHS UK, yes. From September 2024 to June 2025, there were 4,906 calls made to the helpline. The highest number of these calls, about 72%, came from patients and their families. Of all the calls, 2,132 were related to a patient's health getting worse quickly. Of those, 241 calls led to potentially life-saving changes in care, including:
- 93 urgent transfers to an intensive care unit.
- 49 transfers to a different hospital or a higher level of care.
For another 720 calls, the rule led to other important changes, such as starting a new medication like an antibiotic or getting a new scan. This data highlights just how important it is to listen to the concerns of patients and families.
How Martha’s Rule Makes Healthcare So Access?
After the positive results from the first year, the NHS began introducing Martha's Rule to all remaining hospitals in England in April 2025. This includes hospitals that treat adults and children. The program is also being tested in a small number of maternity and mental health units to see if it can be expanded even further.
Professor Meghana Pandit, NHS National Medical Director, said, “There have now been almost 5,000 calls made to the hotlines, with hundreds of potentially life-saving interventions triggered, which is why we are now expanding Martha’s Rule to all acute hospitals in England.”
The health officials acknowledge that, this mistake cost the life of a young girl, but keeping her spirit live through this rule and many others who have used this rule for their loved one. As Health and Social Care Secretary, Wes Streeting said, “No family should ever have to go through what Merope and Paul endured when they lost Martha, but her parents’ tireless campaigning has created a lasting legacy that is already having a potentially lifesaving impact across England.”
CDC Vaccine Panel Gets New Members Again, With A Skeptic In Lead

Credits: Rueters/CDC
The U.S. vaccine advisory landscape is facing a seismic shift as Health Secretary Robert F. Kennedy Jr. moves to reshape the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP). According to an internal CDC document reviewed by Reuters, Kennedy has nominated seven new members to the ACIP, following his controversial ousting of all 17 previous panelists in June. The move has drawn scrutiny from public health experts who fear that vaccine guidance could be increasingly influenced by political considerations rather than scientific consensus.
The ACIP has long been a cornerstone of U.S. vaccine policy, advising the CDC on which vaccines should be administered, to whom, and on what schedule after Food and Drug Administration (FDA) approval. The committee’s recommendations form the basis for insurance coverage across the country and are integral to maintaining herd immunity and public trust in vaccines. Kennedy’s decision to fire all 17 members mid-year and replace them with a hand-picked cohort of eight advisers — one of whom has since left — marks an unprecedented intervention in the advisory process.
Among the seven prospective members named in the latest internal document is Dr. Raymond Pollak, a semi-retired transplant surgeon with a background in immunology. Pollak confirmed that he has been asked to serve on the panel but remains cautious. “I’m being considered pending the vetting process. If I was offered the position, I would think carefully about it,” he told reporters.
Other appointees include Dr. Joseph Fraiman, an emergency medicine specialist in New Orleans; Dr. John Gaitanis, a pediatric neurologist; Dr. Catherine Stein, an epidemiology professor; Hillary Blackburn, a trained pharmacist; and Evelyn Griffin, an obstetrician-gynecologist. None of these professionals were immediately available for comment. Retsef Levi, PhD, will chair the newly restructured COVID-19 Immunization Workgroup, drawing particular concern from infectious disease specialists.
What Are The Concerns Over Panel Independence?
The rapid overhaul of ACIP has sparked widespread debate about the independence and credibility of the panel. Critics argue that Kennedy, a long-standing vaccine skeptic, has moved to stack the committee with individuals whose views align with his own. The firing of CDC Director Susan Monarez last week further amplified these concerns. Monarez reportedly resisted policy changes advanced by Kennedy and was asked to merely rubber-stamp committee recommendations. Her departure prompted resignations from three top CDC officials, citing the secretary’s anti-vaccine policies as their reason.
Does This Pull The Stakes Down For Vaccine Policy in US?
The ACIP is scheduled to meet on September 18, with discussions likely to include recommendations for hepatitis B, measles-mumps-rubella-varicella, and respiratory syncytial virus (RSV) vaccines, according to the Federal Register. Traditionally, the CDC director holds final approval over these recommendations. With the new panel in place, public health experts worry that guidance on critical vaccines — including COVID-19 immunizations — could diverge from established scientific evidence, potentially affecting millions of Americans.
Levi, who will lead the COVID-19 Immunization Workgroup, has previously expressed skepticism about mRNA COVID vaccines. In a 2023 video posted on social media, he asserted that all COVID mRNA vaccination programs “should stop immediately,” claiming that they failed to deliver on efficacy promises and caused “unprecedented levels of harm, including the death of young people and children.” Such statements have alarmed epidemiologists and infectious disease specialists, who warn that this rhetoric could erode public trust in vaccines and heighten vulnerability to preventable diseases.
Implications for Public Health and Vaccine Uptake
Public health experts emphasize that vaccine recommendations are not merely technical decisions; they have real-world consequences for community immunity, disease outbreaks, and healthcare costs. The credibility of ACIP is pivotal in maintaining high vaccination rates, especially for routine childhood immunizations and COVID-19 boosters. Should the panel’s guidance shift toward skepticism of established vaccines, experts fear a rise in vaccine hesitancy, lower immunization coverage, and a resurgence of preventable diseases.
Insurance coverage is closely tied to ACIP recommendations. Altering the panel’s guidance could potentially influence which vaccines are covered by public and private insurers, affecting accessibility and uptake. Given the ongoing threat of seasonal influenza, RSV, and COVID-19, changes to vaccine policy may have profound implications for hospitalizations, morbidity, and mortality, particularly among vulnerable populations such as children, the elderly, and immunocompromised individuals.
Is There Too Much Political Interference in Health Policy-Making?
Kennedy’s actions are part of a broader national debate on the role of politics in public health. While previous ACIP appointments have been largely insulated from political influence, recent interventions signal a shift toward a more politicized approach to vaccine policy. Experts caution that such moves could undermine decades of public trust in the CDC, an institution that has long been regarded as the gold standard in epidemiology and disease prevention.
The revamp of ACIP also underscores tensions within the CDC and the U.S. Department of Health and Human Services (HHS). Multiple top officials have resigned or been replaced in recent months, raising questions about institutional stability and the agency’s ability to respond effectively to public health crises.
The upcoming ACIP meeting on September 18 will be closely monitored by public health officials, epidemiologists, and the media. Key topics likely to be addressed include routine childhood vaccines, COVID-19 booster policies, and emerging infectious diseases such as RSV. Observers will be watching not just the committee’s recommendations but also the process by which they are finalized and approved.
Experts urge the public to remain vigilant and informed about vaccine guidance, noting that evidence-based practices remain critical for preventing disease outbreaks and safeguarding community health. While the political dynamics are evolving, the scientific principles underpinning immunization safety, efficacy, and population protection continue to be essential in maintaining public health.
Robert F. Kennedy Jr.’s appointment of seven new members to the CDC’s ACIP marks a turning point in U.S. vaccine policy. The unprecedented shake-up raises questions about the independence, credibility, and scientific rigor of the nation’s primary vaccine advisory body. With critical vaccine recommendations on the horizon, public health experts, policymakers, and citizens alike are bracing for the potential ramifications of a panel reshaped under political influence, highlighting the fragile balance between science and governance in safeguarding public health.
© 2024 Bennett, Coleman & Company Limited

