- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Credits: Canva
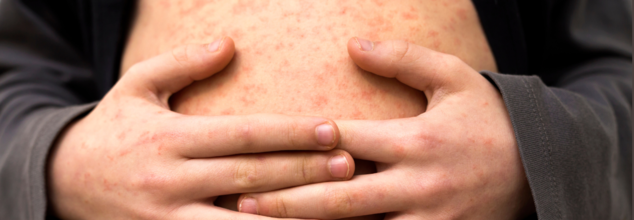
Measles Cases Surge Past 800 in the US, With Half the Country Reporting Outbreaks
The United States is witnessing a dramatic resurgence of measles, a disease once declared eliminated in the country nearly 25 years ago. The Centers for Disease Control and Prevention (CDC) has reported 800 measles cases in 2024 so far, with outbreaks spanning at least 25 states. The alarming uptick comes amid a surge of new infections reported across Texas and neighboring states, prompting renewed calls for aggressive vaccination outreach and public health preparedness.
According to the CDC’s latest weekly update, the national measles count rose by 88 cases in a single week, pushing the year-to-date total to 800. A majority—94%—of these cases are linked to 10 ongoing outbreaks, most notably one sprawling across Texas, New Mexico, Oklahoma, and Kansas.
Texas alone has reported almost 600 outbreak-related cases, and Gaines County has become the epicenter. Altogether, 371 cases have been reported in Gaines County, and 24 other counties throughout the state have also reported infections. New Mexico, Oklahoma, and Kansas have reported 63, 12, and 37 cases, respectively. Michigan and Montana—states that hadn't experienced substantial measles activity in years—are reporting new outbreaks as well, which indicates a disturbing spread beyond initial hotspots.
What is especially alarming about this outbreak is the vaccination history of the infected individuals. CDC reports indicate that an overwhelming 96% of confirmed cases were unvaccinated or of unknown vaccination status. A mere 3% of the confirmed cases had one or two doses of the MMR (measles, mumps, rubella) vaccine. Among the 800 reported cases, a minimum of 85 patients have been hospitalized. There have been three reported deaths, two of them children in Texas. Another death in New Mexico is being investigated.
"Numbers are probably an undercount," experts warn, with many going undiagnosed or unreported—particularly in communities with poor access to healthcare or vaccine resistance.
Texas, and West Texas in specific, is still the hardest hit. The Texas Department of State Health Services (TDSHS) reported 36 new cases after April 15, bringing the total to 597. Upshur County, located in East Texas, has also been a secondary hotspot. TDSHS officials now are looking to see if the cases are part of the initial outbreak or indicate new community-level transmission.
To counter the increasing threat, vaccination centers in regions such as Lubbock—close to the epicenter—have expanded hours and outreach, targeting communities with a history of low vaccination rates.
The U.S. outbreak is not isolated. International travel has been a strong contributor to the reentry and spread of the virus. Multiple Mexican cases have been epidemiologically connected to the outbreak in Texas, and three Colorado cases are under investigation following travel to Mexico. A Pennsylvania resident, meanwhile, was diagnosed with measles after travel to Texas, though the specific site of exposure is unclear.
Canada's persistent outbreak, specifically in Ontario, also fueled the spread, as Michigan authorities traced Montcalm County cases back to Canadian infections. Such cross-border linkages underscore the imperative for worldwide containment of measles and strong immunization systems across the globe.
What is Driving US Measles Outbreak?
Measles is also a very contagious airborne disease, transmitting by sneezing and coughing. An infected person alone can infect 90% of non-vaccinated individuals in the vicinity. The virus even lasts in air and on surfaces for two hours after an infected person has already exited the room.
Health authorities attribute the epidemic of measles to:
Lowered Vaccination Levels – Most of the recent cases have been seen in populations with lower than recommended measles-mumps-rubella (MMR) vaccination coverage. Some parents continue to refuse or postpone vaccination, putting their children and populations at risk.
Global Travel – Measles is more common in parts of Africa, Asia, and Europe, and unvaccinated travelers can bring the virus to the U.S.
Delayed Medical Treatment – The majority are undiagnosed or not reported in the early stages, leaving the virus unchecked.
A Threat to Measles Elimination Status
The United States of America officially eliminated measles in 2000, when uninterrupted transmission of the virus had lasted for more than a year. But as cases increase, the status of the country's elimination is threatened.
"That … would occur after 12 months of continuous circulation of the same strain," said Dr. David Sugerman, a senior CDC scientist, at a recent advisory session. If the virus keeps circulating through early 2026, the U.S. may forfeit its measles elimination status a major public health loss.
What You Must Know About Measles?
Measles is preceded by the symptoms of fever, cough, runny nose, and reddish watery eyes. Then comes a typical red rash starting at the hairline and radiating downward.
The virus is among the most infectious illnesses that have ever affected human beings. A contaminated individual can infect as many as 90% of unprotected people around him or her, and the virus will remain airborne and on surfaces for as long as two hours after infection.
The MMR vaccine is still the best prevention. Two doses provide around 97% protection. The CDC encourages everyone particularly children, healthcare providers, and travelers abroad, to stay current on their vaccinations. Unvaccinated travelers must be vaccinated at least two weeks prior to travel to prevent possible exposure overseas.

Credits: Canva
World Malaria Day 2025: Theme, History, And Significance
Every year on 25 April, World Malaria Day is observed to raise awareness about one of the deadliest yet preventable diseases. As per the World Health Organization (WHO), there were 263 million malaria cases and 5,97,000 malaria deaths across 83 countries in 2023. The WHO African Region carries a disproportionately high share of the global malaria burden. As per the numbers, this Region was home to 94% of the malaria cases, accounting for 246 million and 95% of malaria deaths. Children under 5 accounted for about 75% of all malaria deaths in the Region.
Ahead of World Malaria Day, WHO also called for renewed efforts at all levels - from global policy to community action to accelerate progress towards eliminating malaria.
World Malaria Day 2025 Theme
This year, WHO has joined the RBM Partnership to End Malaria and other partners in promoting: "Malaria Ends With US: Reinvest, Reimagine, Reignite". This is a grassroot campaign that aims to re-energize efforts at all levels, from global policy to community action, to accelerate progress towards malaria elimination.
World Malaria Day History
World Malaria Day was first celebrated internationally in 2008, building upon the earlier "Africa Malaria Day", which had been observed b African countries since 2001. The date, April 25, was established by WHO in 2007 during the World Health Assembly. In 2007, it was the 60th session of the World Health Assembly where the proposal to rename Africa Malaria Day to World Malaria Day was made to acknowledge the global presence of malaria.
World Malaria Day Significance
The day has a strong significance in healthcare as it brings attention to the disease that still continues to take so many lives, especially in low-income and tropical regions. It also serves as an important reminder to continue spreading awareness about the disease as well as promoting its prevention, treatment and continuous international cooperation to fight against it.
What Is Malaria?
The WHO describes malaria as a life-threatening disease spread to humans by some types of mosquitoes, mostly found in tropical countries. However, they are preventable and curable.
WHO notes: "Malaria is spread to people through the bites of some infected anopheles mosquitoes. Blood transfusion and contaminated needles may also transmit malaria. The first symptoms may be mild, similar to many febrile illnesses, and difficult to recognize as malaria. Left untreated, P. falciparum malaria can progress to severe illness and death within 24 hours.
There are 5 Plasmodium parasite species that cause malaria in humans, and 2 of these species – P. falciparum and P. vivax – pose the greatest threat. P. falciparum is the deadliest malaria parasite and the most prevalent on the African continent. P. vivax is the dominant malaria parasite in most countries outside of sub-Saharan Africa. The other malaria species which can infect humans are P. malariae, P. ovale and P. knowlesi."
What Are The Symptoms?
The early symptoms are fever, headache and chills, which can usually start within 10 to 15 days of getting bitten by an infected mosquito.
Some types of malaria can cause severe illness and death. Infants, children under 5 years, pregnant women, travellers and people with HIV or AIDS are at higher risk. Severe symptoms include:
- extreme tiredness and fatigue
- impaired consciousness
- multiple convulsions
- difficulty breathing
- dark or bloody urine
- jaundice (yellowing of the eyes and skin)
- abnormal bleeding
Credit: Canva
Blood Test That Can Detect 12 Types Of Cancer To Go On Trial In UK
A new AI-powered blood test that can detect 12 types of cancer is to be tested on NHS patients. Using this test, experts would now be able to detect cancerous cells in people much before the symptoms appear. The trial, conducted on 8,000 patients, will analyse blood samples for tiny fragments of genetic material released by tumours.
The test called miONCO-Dx, was created using data from 20,000 patients. Initial tests have produced promising results, having shown that it can detect 12 of the most lethal and common cancers, including bowel cancer, at an early stage, with over 99% accuracy. With no other trial currently working in the same way, this is a world-leader and will support in placing Britain at the forefront of revolutionising healthcare. Notably, the UK government has awarded £2.4m to run the trial of the genetic test, which was developed by the University of Southampton and the biotech startup Xgenera.
How Does It Work?
The test was created by Xgenera, in collaboration with the University of Southampton. As little as 10 drops of blood are all that's needed to detect up to 12 common cancers. The test works by measuring the microRNA in a blood sample and using AI to identify if cancer is present and, if so, where it is located in the body.
What Cancers Will It Detect?
Lung, gastric, prostate, oesophageal, liver, bladder, ovarian, bowel, pancreatic and breast cancers–as well as bone and soft tissue sarcoma and a type of brain tumour. The Department of Health said the test was now ready for the "validation and verification" stage.
Professor Sir Stephen Powis, NHS England's national medical director, said: "This blood test has the potential to help us detect bowel cancer earlier and reduce the need for invasive tests, and the next step in this trial will now be vital in gathering further evidence on its effectiveness and how it could work in practice."
How Is A Blood Test Conducted?
A blood test is a simple medical procedure used to check various health conditions. Here's how it is typically conducted:
1. Preparation: Depending on the test, you may be asked to fast for 8–12 hours. The healthcare provider will explain any specific instructions.
2. Collection: You’ll usually be seated or lying down. A healthcare professional will tie a tourniquet around your upper arm to make the veins more visible. The inside of your elbow is the most common site for drawing blood.
3. Cleaning: The area is cleaned with an antiseptic to reduce the risk of infection.
4. Drawing Blood: A sterile needle is inserted into the vein, and blood is collected into one or more vials or tubes.
5. Post-collection: Once enough blood is collected, the needle is removed, and a cotton ball or bandage is applied to stop any bleeding.
6. Processing: The blood samples are then sent to a lab for analysis.
Credits: Canva
Novavax Says FDA Approval Back on Track for Its COVID Vaccine
Novovax, the maker of the only protein-based COVID-19 vaccine available in the US announced that its shot is on track for full approval from the US Food and Drug Administration (FDA). It is an important development for the company. It has sent its stock soaring up to 21% on Wednesday morning for trading. It is said that this will also ease the fears of political interference that may have caused delay in the process.
Vaccine For Emergency Use Only
While the other mRNA vaccines from Pfizer and Moderna have received full FDA approval for specific age groups, Novovax's vaccine still awaits the approval. It is only authorized for emergency use.
The emergency use authorization or the EUA allows vaccines to be distributed during public health emergencies. However, once the emergency ends, the FDA can remove them from the market unless full approval is granted.
Why Did The Delay Happen?
The FDA originally planned to approve Novovax's vaccine by April 1. However, as per the inside sources, the process was paused at the direction of Dr Sara Brenner, the FDA's acting commissioner. The delay has also raised concerns, especially after Dr Peter Marks, the FDA's longtime vaccine chief, reportedly left his post due to disagreements with the Health Secretary Robert F Kennedy Jr, who is a known vaccine skeptic.
What Makes Him A Vaccine Skeptic?
In the past, RFK Jr. has worked closely with many anti-vaccine activists who work for his nonprofit group Children's Health Defense. While in his recent speech, he said that he has "never been anti-vax and have never told the public to avoid vaccination", his track record shows otherwise.
In a podcast interview, he said, "There is no vaccine that is safe and effective" and told FOX News that he still believes in the now long-debunked idea that vaccines can cause autism. In another 2021 podcast, he urged people to "resist" CDC guidelines on getting their kids vaccinated. "I see somebody on a hiking trail carrying a little baby and I say to him, better not get them vaccinated," he said.
His non-profit also led an anti-vax campaign sticker and he appeared next on the screen to a sticker that read: "If you are not an anti-vaxxer you are not paying attention," reports AP.
What Does The Vaccine Need For Approval?
The FDA recently asked Novavax to outline a plan to collect additional clinical data from people who have received the vaccine. Novavax says it is “engaging with the FDA expeditiously” and hopes to secure full approval as soon as possible. Full FDA approval is considered the gold standard, as it reflects a higher level of scrutiny and confidence in a product’s safety and effectiveness.
How Is This Vaccine Different From Others?
The COVID-19 vaccines that are currently available in the US teach the immune system to recognize the virus' spike protein, which is its outer coating. the Pfizer and Moderna's mRNA vaccine deliver genetic instructions that help the body create a temporary version of spike protein that trigger an immune response. In contrast, the Novovax's shot contains lab-grown copies of the spike protein itself, which are then combined with a substance that boosts the immune response.
This traditional approach—called a protein-based vaccine—has been used for decades in vaccines for diseases like hepatitis B and shingles. For people who are hesitant about mRNA vaccines, Novavax offers an alternative that uses a well-established method.
© 2024 Bennett, Coleman & Company Limited

