- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Measles Outbreak: North Carolina Confirms Its First Case Of 2025
Credits: Canva
Amid the ongoing measles outbreak in the United States, the state of North Carolina has confirmed its first case in 2025. The North Carolina Department of Health and Human Services (NCDHHS) confirmed a case of measles in a child who was visiting Forsyth County and Guilford County.
How Did The Child Fall Sick?
The child became ill while traveling to North Carolina from a region where measles had already been reported. The news is confirmed as per the NCDHHS release.
The authorities are now also asking people who have visited the below mentioned location to review their immunization records or contact a healthcare provider to ensure that you are updated with the measles-mumps-rubella vaccine.
The Locations Are:
- PTI Airport in Greensboro
- Sleep Inn, 1406 Heartland Dr., Kernersville
- McDonalds, 14000 Heartland Dr., Kernersville
- Greensboro Science Center, 4301 Lawndale Dr., Greensboro
- Ice cream shop at Piedmont Triad Farmers Market, Greensboro
- Greensboro Aquatic Center Foyer, 1921 W. Gate City Blvd., Greensboro
- Greensboro Partee Shack, 3712 S. Holden Rd., Greensboro
- Lowes Foods, 240 Market View Dr., Kernersville
What Are The safety Guidelines?
The NCDHHS has also recommended that anyone unvaccinated and older than one year must get the measles vaccination.
However, when it comes to lab tests, the NCDHHS recommends that it is not necessary for people who were exposed, unless they develop symptoms of measles, including fever and rash. These symptoms can start from 7 to 21 days of getting infected. “Getting vaccinated against measles continues to be the most important step we can take to protect ourselves and our loved ones,” NCDHHS Secretary Dev Sangvai said. “It is important to check with your health care provider to ensure you are current with all your vaccines.”
What Is Measles?
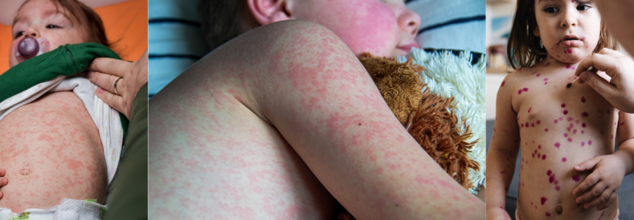
As per the Centers for Disease Control and Prevention (CDC), measles is a highly contagious disease that comes with rashes and are especially prone to kids under the age of 5.
If one person gets it then 9 out of 10 people around the infected person may become infected.
Measles, also known as rubeola, is one of the most contagious infectious diseases, with at least a 90% secondary infection rate in susceptible domestic contacts. It can affect people of all ages, despite being considered primarily a childhood illness.
Measles is marked by prodromal fever, cough, coryza, conjunctivitis, and pathognomonic enanthem (ie, Koplik spots), followed by an erythematous maculopapular rash on the third to seventh day.
Who Is At Risk?
The World Health Organization (WHO), calls measles as one of the world's most contagious disease, which is spread by contact with infected nasal or throat secretions through coughing or sneezing, or breathing the air that was breathed by someone with measles. The virus remains active and contagious in the air or on infected surfaces for up to two hours.
WHO notes that any non-immune person (not vaccinated or vaccinated but did not develop immunity) can become infected. Unvaccinated young children and pregnant persons are at highest risk of severe measles complications.
Not Wegovy or Mounjaro Or Ozempic: This New Monthly Weight Loss Jab Helped People Drop 20% Body Fat
Credits: Canva (representational)
A new clinical trial has uncovered the breakthrough promise of a new weight loss medication, MariTide, that can be taken only once monthly. Amgen developed the investigational drug, and it has demonstrated remarkable weight loss outcomes up to 20% in individuals with obesity. The Phase 2 trial results, which were released in The New England Journal of Medicine and presented at the American Diabetes Association's annual meeting 2025, may be a turning point in the treatment of obesity and Type 2 diabetes worldwide.
MariTide is a GLP-1 receptor agonist, a type of drug whose behavior is well-known to reduce weight and control Type 2 diabetes. It differentiates from current competitors like Novo Nordisk's Ozempic and Wegovy, and Eli Lilly's Mounjaro and Zepbound, because it has a distinct molecular structure. MariTide contains a monoclonal antibody that means the drug can remain in the body longer. This capability makes the drug possible to give as a once-monthly injection, a major improvement over its weekly-injected counterparts.
Amgen's Phase 2 clinical trial consisted of almost 600 adults, divided into two groups: one with obesity alone and the other with obesity and Type 2 diabetes. Volunteers were assigned to take one of three dose levels of MariTide or a dummy shot. They took monthly injections for 52 weeks, and some in the obesity group started with a low dose that was increased incrementally.
The findings from the Phase 2 clinical trial of MariTide were nothing short of remarkable. Participants who had obesity but did not have diabetes experienced an average weight loss of up to 20% of their body weight after 52 weeks of treatment. Meanwhile, those who were managing both obesity and Type 2 diabetes saw an average weight loss of up to 17%. In sharp contrast, patients in the placebo groups showed only slight decreases in body weight, 2.6% for the group with obesity alone and only 1.4% for the group with obesity and diabetes. These findings highlight the great potential of MariTide as a very effective, once-a-month weight loss treatment.
These findings place MariTide on par with Wegovy and Zepbound in weight loss effects. Although Wegovy studies recorded a 15% decrease after 68 weeks and Zepbound delivered a 22.5% decrease after 72 weeks, MariTide's monthly regimen and similar effectiveness make it a strong contender.
Health Benefits of Using MariTide
MariTide is not only gaining recognition for its effectiveness at weight loss—it's also emerging as a full-range cardiometabolic therapy. Based on trial results, patients on MariTide experienced a dramatic decrease in A1c values, a key measure of long-term blood glucose control. For patients with diabetes, the medication reduced A1c by as much as 2.2 percentage points, outpacing expected decreases of 1.5 to 2.0 points with established GLP-1 therapies such as Ozempic and Mounjaro. But the advantages didn't end there. The research also observed significant reductions in blood pressure, cholesterol, and markers of inflammation, pointing to the potential of MariTide beyond the scale. These results indicate that the drug may provide an all-around solution to obesity as well as other chronic diseases such as cardiovascular disease and type 2 diabetes, making it an attractive option in the changing scenario of metabolic health treatments.
How MariTide Works?
Similar to other GLP-1 receptor agonists, MariTide has a similar action to glucagon-like peptide-1, a hormone that controls blood glucose and hunger. It increases the release of insulin, decreases the release of glucagon, and retards gastric emptying. MariTide differs from other drugs in that it contains a monoclonal antibody that keeps the drug in the body longer. Monthly dosing is made possible by this advance, and treatment compliance may be enhanced by less frequent injections.
Side Effects of 'MariTide' That People Need to Know
Similar to other GLP-1 drugs, side effects in the gastrointestinal tract were most frequently reported during the trial. These consisted of:
- Nausea
- Vomiting
- Diarrhea
- Abdominal discomfort
The side effect profile was comparable to other drugs in the same class according to the research team and largely tolerable overall. Large-scale Phase 3 trials will be important to best evaluate long-term safety and infrequent adverse events, though.
MariTide vs Wegovy, Mounjaro, and Others
If approved, MariTide would represent Amgen's foray into a weight loss market led by Novo Nordisk and Eli Lilly. Wegovy and Mounjaro have experienced explosive growth as a result of their robust efficacy results. Yet, MariTide's monthly dosing regimen and dual utility in diabetes care and weight loss may resonate with a vast patient population.
The comparisons between MariTide and existing GLP-1 drugs have not yet been conducted. Still, the early data suggests that Amgen’s candidate could stand toe-to-toe with the industry giants.
What’s Next for MariTide?
Amgen said it plans a Phase 3 clinical trial to further study MariTide for 72 weeks. If all goes well, the results of this research may lead to FDA approval and future distribution worldwide. Most analysts expect it will be at least a couple of years before MariTide is widely available, though.
With rates of obesity on the rise globally and related health issues costing billions each year, effective, affordable, and sustainable weight-loss solutions have never been more vital. MariTide's encouraging results provide a timely new alternative to an expanding stockpile of obesity-fighting weapons.
Novo Nordisk Launches Bestseller Weight Loss Drug Wegovy In India
Credits: Novo Nordisk
Global pharma giant Novo Nordisk launched its blockbuster weight loss drug Wegovy in India, a pivotal moment in the battle against increasing obesity levels in the nation. This follows closely on the heels of Eli Lilly's Mounjaro launch in India.
Wegovy, the semaglutide-based GLP-1 receptor agonist, will be available by the end of the month across major pharmacy chains in India. The once-weekly injectable prescription drug has already changed the landscape of obesity management in the United States and Europe.
"One out of every three patients attain 20% weight loss using the increased dose of Wegovy," stated Vikrant Shrotriya, Managing Director, Novo Nordisk India. The firm has completed late-stage trials involving 3,500 people in India, indicating encouraging results in sustained weight loss.
Wegovy will come in several dosages, ranging from Rs 4,336 for 0.25 mg to Rs 26,015 for the highest 2.4 mg dose. Its price is comparable to its rival Mounjaro, which was also launched in India last month in March.
Wegovy is timely given the increasing weight problem in India. A recent National Family Health Survey reveals that 24% of Indian women and 23% of Indian men aged between 15 and 49 years are overweight or obese—a notable increase from past years. India, with a population of over 1.4 billion and fast-emerging lifestyle diseases, is a huge market for weight-loss medications.
The medication has already proven to be successful across the world. Clinical trials have indicated that Wegovy consumers would lose around 15% of their body weight on average, especially when combined with a balanced diet and regular exercise. For comparison, Mounjaro (tirzepatide), being a dual GIP and GLP-1 agonist, experienced close to 23% of weight loss under the same circumstances.
Novo Nordisk's launch in India is at a time of corporate leadership transition, following the recent resignation of CEO Lars Fruergaard Jorgensen. The move is said by analysts to be intended to protect market share in a competitive environment rising from Lilly competition as well as Indian generic drugmakers who are gearing up to move into the field of obesity drugs when semaglutide's patent is about to end in 2026.
Although Wegovy brings new hope to millions of people with obesity, there are warnings with the medication. The drug has a boxed FDA warning about the potential for thyroid cancer, as suggested by studies on animals. Because of this, the drug is not approved for use in someone with a personal or family history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia syndrome type 2 (MEN2).
As India joins the global fight against obesity with cutting-edge therapies, India is all set to benefit from Novo Nordisk's Wegovy, which is ready to provide a new, science-driven solution for long-term weight management. The competition has already turned hot, and for Indian consumers, it could mean more options, improved results, and a new age of obesity treatment.
15-Pound Newborn Shocks Hospital Staff, Doctors Say It's a 1 in 10,000 Birth

(Credit-Stacie Golebiowski)
When Stacie Golebiowski went for her 36-week ultrasound last summer, she knew her baby would be big, but no one was truly ready for just how big. On July 4, 2024, at Orillia Soldiers’ Memorial Hospital (OSMH), Stacie gave birth to her son, Grayson, who weighed a surprising 15 pounds and 10 ounces. This was such an unusual size that it caused a first-ever problem for the hospital staff when trying to record his weight.
System Limitations Of Dealing With Above Average Baby Weight
Laura Ferris, who helps manage women and children's services at the hospital, explained that when Grayson was born, the nurses found his weight was too high for the Ontario registry system. They actually had to call the registry, which then had to manually update their records – something that had never happened before.
Now, as Grayson gets close to his first birthday, he's doing great. Stacie shared that there were difficulties and unknowns with Grayson at the start, even for the doctors and nurses. But they got through it all, and he's "doing amazing."
The family hopes their story can help other parents who have unusually large babies. Stacie mentioned feeling like she might have done something wrong, but she emphasized that it's important for parents to understand that it's just what their body does and it's not their fault. According to BORN Ontario, Grayson's birth weight puts him in the top 0.01 percent of all babies born in the province.
Concerns Regarding High Birth Weight
According to MedlinePlus, when babies are born larger than average, it's called having a high birth weight. While every baby is a blessing, being very big can sometimes come with a few challenges. For example, it can make the delivery more difficult for both the baby and the mother, and there might be a higher chance of minor injuries during birth. After birth, bigger babies might also face some specific health issues like,
Blood Sugar Issues
Their bodies might have trouble keeping their blood sugar levels steady, which needs to be watched closely by doctors.Breathing Problems
Sometimes, these babies can have trouble with their breathing, requiring extra care.
Jaundice
This is a common condition that causes a yellowing of the skin and eyes because of too much bilirubin in the blood. While many babies get jaundice, those with a high birth weight might be more prone to it or have a more noticeable case.
Risks Associated with Higher Birth Weights
The Standford Medicine Children’s Health points out that many large babies are born to mothers with diabetes. They also list the risks that are associated with high birth weight,
- A long labor.
- A difficult birth.
- Injuries to the baby, such as a broken collarbone or nerve damage in the arm.
- A greater chance of needing a C-section.
- Higher risk of birth defects.
Does High Birth Weight Cause Health Issue?
According to a 2021 study published in the Frontiers in Pediatrics journal, more and more children are being born with high birth weight or are considered "large for gestational age" (LGA). This is happening even more with babies conceived through certain fertility treatments like frozen embryo transfer (FET). While we know a lot about the short-term risks for these babies, less is known about their health in the long run.
A 2021 study looked at the connection between high birth weight and long-term health in children. After reviewing many studies, it found that being born with a high birth weight or LGA was linked to a slightly higher risk for certain cancers in childhood (including breast cancer), some mental health conditions, high blood pressure in childhood, and both type 1 and type 2 diabetes.
© 2024 Bennett, Coleman & Company Limited

