- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
WHO Recommends New HIV Twice-Yearly Shot Amid Fund Cuts; What Are The Big Challenges The Drug Faces?
Credits: Health and me
In what could be a transformative moment in the fight against HIV, the World Health Organization (WHO) has officially recommended the use of injectable lenacapavir—a long-acting antiretroviral medication—in the prevention of HIV. The news comes barely weeks after the U.S. Food and Drug Administration (FDA) approved lenacapavir as a twice-yearly injection for PrEP (pre-exposure prophylaxis).
Timing is everything, in 2024 alone, an estimated 1.3 million new HIV cases were reported globally, disproportionately falling on populations already suffering from stigma and barriers to healthcare. From Kigali to California, this new strategy has the potential to change the delivery of HIV prevention—and to whom.
The medication lenacapavir acts on the HIV capsid, or virus protein shell, inhibiting its capacity to make copies of itself and infect new cells. Its uniqueness lies in the fact that it has a long half-life and is effective at low doses. A single injection administered subcutaneously establishes a reservoir of drug that gradually delivers medication into circulation for 26 weeks.
This enables dosing every two years, a huge benefit for individuals who might not be able to manage daily oral PrEP because of lifestyle, access, or stigma. As WHO Director-General Dr. Tedros Adhanom Ghebreyesus said, "While an HIV vaccine remains elusive, lenacapavir is the next best thing."
The historic guidelines, released at the International AIDS Society Conference in Kigali, Rwanda, encourage governments and health systems to implement this new tool, especially in high-incidence settings and among priority groups such as sex workers, men who have sex with men, transgender people, people who inject drugs, and adolescents.
The Evolution of HIV Prevention
Since the discovery of HIV in the 1980s, prevention has been held on three pillars: biomedical progress, barrier methods, and behavioral change. The early decades were dedicated to encouraging condom usage, lowering high-risk behavior, and public awareness. These strategies slowed but did not curb the virus.
In 2010, a new era opened up. Clinical trials revealed that antiretroviral drugs not only treated HIV but could also prevent it. This led to widespread adoption of PrEP, typically in the form of daily oral pills. Meanwhile, the concept of U=U (Undetectable = Untransmittable) proved that treatment with good adherence blocked transmission altogether.
By 2019, PrEP had been given an "A" rating by the U.S. Preventive Services Task Force, resulting in its coverage without out-of-pocket expense. Despite all these developments, the HIV epidemic has continued, with more than 30,000 new infections reported every year in the U.S. alone.
Why Daily PrEP Isn't Enough?
PrEP taken daily is effective, but it has conditions. It needs to be used every day consistently, tested regularly for HIV and STIs, and have a reliable healthcare system in place. For most, these are difficult requirements to fulfill.
Individuals most vulnerable to these challenges, such as people of color, LGBTQ+ persons, low-income individuals, and those in the southern United States, frequently experience substantial barriers to daily PrEP maintenance. These barriers often involve lack of insurance, transportation, stigma, and health system distrust.
Injectable lenacapavir bypasses several of these obstacles. Just two shots annually, it provides an inconspicuous, low-fuss choice that might significantly boost takeup among under-served communities.
The breakthrough arrived with the PURPOSE 1 trial, which took place in South Africa and Uganda. More than 5,300 young women and adolescent girls, among the most at-risk populations in the world, were recruited.
The results were astonishing: zero new infections in participants who received lenacapavir, compared with 39 and 16 infections in two oral PrEP control groups. The background incidence rate was 2.41 per 100 person-years. In the world of HIV prevention, this is as close to a vaccine-level efficacy as we’ve ever seen.
How Funding Crisis Threatens Momentum?
As the excitement takes hold, a new challenge is on the horizon. UNAIDS warns that a huge funding gap threatens to undermine global HIV gains. The U.S. President's Emergency Plan for AIDS Relief (PEPFAR), which had committed $4.3 billion to 50 nations in 2025, suddenly suspended funding earlier this year.
Already, the effect can be seen. In Nigeria, access to PrEP fell from 40,000 recipients to fewer than 7,000 within months. Kenya documented dramatic drops in women receiving preventive treatment after giving birth. "This is not a funding gap—it's a ticking time bomb," UNAIDS Executive Director Winnie Byanyima said. "Health workers are being sent home. Services are disappearing overnight."
The WHO is banking on lenacapavir's ease and extended dosing interval to reverse some of this backlash by lowering costs per patient and alleviating pressures on overwhelmed systems. But to be realized, its promise will have to be matched by global cooperation and domestic investment.
With WHO backing, FDA approval, and compelling trial data, lenacapavir is poised to become a pillar of modern HIV prevention. Countries must now prioritize fast-tracking its regulatory approval, training providers, and ensuring equitable access.
It's not about a new drug. It's about giving individuals more control of their sexual health, providing options that work in real lives, and ending the gap between what we know we can do and what people end up getting.
The global HIV epidemic is far from over. But with tools like lenacapavir, we are getting closer to turning the tide—and protecting the next generation from a virus that has shaped public health for over four decades.
Scientists Just Fixed A 'Faulty' Gene Behind Deafness And Hearing Loss In Children
Credits: Credits
For generations, congenital deafness has been a lifetime of silence or partial hearing regained by way of invasive procedures such as cochlear implants. As revolutionary as those devices have been, however, they have their limitations—especially for people who are born with genetic types of hearing loss that destroy the natural processes of sound conduction but a new scientific advance is now making something once considered impossible a possibility- actually restoring natural hearing through gene therapy.
First-of-a-kind, researchers have been able to restore hearing in youngsters, teens, and even young adults who were born with a certain form of congenital deafness. The therapy, which aims at an errant gene called OTOF, is a landmark in the management of genetic hearing disorders.
What Is OTOF-Related Deafness?
The culprit gene, OTOF, encodes a protein known as otoferlin, which is vital for the conduction of sound signals from the inner ear to the brain. When the gene is mutated, the otoferlin protein is either absent or defective, and the person experiences severe hearing loss since birth. The inner ear itself is normal—only the signaling system is defective.
That's precisely why OTOF-associated deafness is such a strong candidate for gene therapy. If you can restore or replace the defective gene, the body already has the physical equipment in place to naturally process sound.
The research team, based in five hospitals and spearheaded by genetic hearing loss experts, employed a virus that has been altered as a delivery vehicle. This virus was altered to transport a healthy version of the OTOF gene and inject it directly into the hair cells of the inner ear—the sound-detecting sensory cells.
This is how it works: the genetically altered virus binds to a hair cell surface, enters the cell, and delivers the repaired gene into the nucleus. The cell then starts manufacturing working otoferlin protein, effectively rewiring the ear-to-brain communication line. It's not science fiction—this is precision molecular medicine in action.
The most impressive feature of this research is its wide range of participant ages, ten people between 1 and 24. All ten of them had OTOF-related congenital deafness. It was the first research study to consider whether gene therapy might cure hearing in children and young adults—not only in babies or toddlers.
The treatment consisted of a single injection of the virus carrying the gene into the inner ear of the patient. Patients were tested for 12 months with both objective hearing measures (such as auditory brainstem responses) and behavioral tests (like button-pushing in response to hearing specific tones). Results were immediate and dramatic. During the initial month, patients showed:
- A 62% increase in brainstem hearing response tests
- A 78% increase in behavioral hearing tests
- In a few instances, near-normal speech perception
A seven-year-old patient reportedly started reacting to sounds only three days post-treatment—a moment that surprised the medical staff and the family alike.
Even more promising, the side effects were minimal and easy to manage, the most frequent being a brief fall in white blood cell level. No severe adverse events were noted, affirming the safety of this gene therapy strategy.
How Does Gene Therapy Works?
Interestingly, the research found that 5- to 8-year-old children gained the most—more than toddlers. This contradicts earlier beliefs that earlier is always better in cases of congenital conditions. It implies that the brain's preparedness to receive new auditory information could be as crucial as the ear's structural integrity.
Why exactly this age window is optimal is still unclear, but it's an important question for future scientists to answer as they continue to fine-tune the timing of treatments.
This is not merely another clinical milestone, it's a shift in paradigm. By addressing the root genetic cause of deafness, this therapy holds the potential for a one-time fix that restores hearing naturally without the implant or the lifetime devices.
And although this trial involved the relatively uncommon OTOF mutation, the implications are far-reaching. Researchers are currently investigating gene therapies for other, more prevalent forms of inherited deafness with complicated patterns of genetics. Promising early results have been seen in animal studies, and human trials may follow in the next few years.
Additional information will be required to ascertain for how long the restored hearing lasts. Will the effects last the rest of one's life, or will there be the need for booster shots? Long-term follow-up studies are already in process.
Meanwhile, this achievement is a beacon of hope for families who suffer from genetic deafness—and a strong reminder that the future of medicine is not about compensating for our genes but rather about correcting them.
For the first time, gene therapy has shown that it can restore hearing in individuals born with silence—not only control their condition, but change it. The more this research grows and technology develops, the world is moving closer to a time when congenital deafness could one day not only be treated, but cured and for families with genetic hearing loss, that's not only science—it's life-altering.
PCOS May Be Passed Down Through ‘Epigenetic Memory’ Even Before You Are Born—Could This Genetic Discovery Help Prevent It?

Credits: Health and me
Scientists may have discovered a critical clue that helps explain why polycystic ovary syndrome (PCOS)—a hormonal condition affecting up to 13% of women of reproductive age—tends to run in families. New research presented at the 41st Annual Meeting of the European Society of Human Reproduction and Embryology in Paris suggests that PCOS may be passed down not just through classic genetics, but through what’s known as epigenetic memory.
In simple terms, epigenetics is chemical change that affects how genes are switched on or off without actually changing the DNA sequence itself. These changes can be caused by environment, stress, and diet and more importantly, they can occasionally be passed on. This throws open the gate for a whole new perspective of PCOS as not only a genetic disorder but an epigenetic disorder, possibly heritable from mother to daughter through early cellular processes.
PCOS is characterized by an extensive array of symptoms that can significantly affect quality of life. They encompass irregular or heavy menstrual periods, ovarian cysts, weight gain, acne, hirsutism, thinning hair, and infertility. The disorder also elevates the risk of developing metabolic complications like hypertension, type 2 diabetes, and endometrial cancer.
Despite decades of studies, the underlying cause of PCOS has not been determined. Nonetheless, it is firmly established that the condition usually has an inherited pattern. PCOS is estimated to affect 20% to 40% of patients who have one affected mother or sister, and findings from twin studies—such as the Dutch Twin-Family Study—indicate a very strong genetic basis.
The research, led by Dr. Qianshu Zhu of Chongqing Medical University in China, explored the epigenetic signatures in egg cells and embryos from women who were receiving in vitro fertilization (IVF). Of the 230 women researched, 133 had PCOS while 95 did not.
The scientists tested both unfertilized egg cells and early embryos for epigenetic markers—chemical labels that perch on DNA and control gene expression. What they discovered was dramatic: egg cells and embryos from PCOS women had pervasive disturbances in important genes, especially those related to metabolism and early embryo growth.
Also, they found abnormalities in an epigenetic marker called H3K27me3, which is important for gene expression. These abnormalities were present in the egg cells and were inherited by the embryo—indicating that epigenetic signals might be inheritable, even prior to the onset of implantation.
This indicates that an epigenetic message is being transmitted from mother to embryo prior to implantation even starting," said Dr. Zhu. A woman's gene expression related to PCOS, in other words, might be pre-programmed into the development of the embryo from day one.
What Is Epigenetic Inheritance Exactly?
Whereas genetic mutations entail alterations in the DNA sequence itself, epigenetic inheritance refers to the passing on of gene expression patterns from one generation to the next. Such changes are initiated by lifestyle elements, but once they take root, they could be "remembered" by cells—and transmitted to offspring.
If these results hold up to further study, it may be that PCOS is inherited, at least in part, by epigenetic memory, which would explain its familial occurrence even in the absence of identifiable genetic mutations.
Dr. Sydney Chang, a fertility specialist and medical director of CCRM Fertility of Austin, said that the new discovery provides an explanation for why identical twins (who have 100% DNA in common) are much more likely to both develop PCOS compared to fraternal twins. "This indicates that much of the risk of developing PCOS is inherited by genes," she said—but possibly not so-called "traditional" genes.
Implications for IVF and Reproductive Health
The possible uses of this work are both thrilling and morally complicated. Dr. Zhu's lab implies IVF technology may eventually involve screening or intervening on the epigenetic level to prevent PCOS—perhaps by fine-tuning the gene expression of embryos prior to implantation—a frontier currently years from use in clinics, but in theory possible.
Also, this study may have an impact on how fertility clinics select embryos and further our knowledge of the direct influence of maternal health on early embryonic development.
But Zhu was quick to caution: these are laboratory-created embryos, not kids. His lab now is carrying out mouse studies to observe how these epigenetic alterations impact real offspring.
Why Early Detection Of PCOS Is Important for Women Health?
One of the most exciting implications of this research is that it has the potential to induce early diagnosis and treatment. Eventually, the findings may enable clinicians to identify risk of PCOS earlier in life—even prior to the onset of symptoms.
What is interesting about this research is that it validates a true genetic link among PCOS in families, It sheds light for early diagnosis and preventive interventions to keep PCOS from being passed through families.
In the future, this could translate into lifestyle or medical interventions that alter epigenetic expression before the condition fully develops, opening new doors in both prevention and personalized care.
Although the research provides an important new perspective on PCOS, it also brings as many questions as answers. How do those epigenetic changes cross-talk with environmental influences such as diet, stress, or hormonal exposure? Can they be undone later in life? Are they specific to PCOS, or can they have a role in other hormonal conditions as well?
Dr. Zhu's continued research in animal models will help work through these unknowns, but longitudinal studies and human clinical trials will be essential to translating the laboratory findings into actual practice.
PCOS has long baffled scientists and plagued patients with imprecise diagnoses, capricious symptoms, and too often, delayed treatment. But as new research points toward an epigenetic memory with risk that gets passed across generations, the disease may soon be explained not just as a hormonal imbalance—but as a biological inheritance that contemporary medicine can detect more effectively and one day, conceivably, interrupt.
Will This Genetic Breakthrough Prevent PCOS?
Yes, this gene find may open the door to possible prevention, but with significant qualifications. The research unveiled at the European Society of Human Reproduction and Embryology indicates that polycystic ovary syndrome (PCOS) might be affected not only by genes that are passed down, but by epigenetic memory—chemical modifications to DNA that influence the activation or deactivation of genes. Scientists discovered these epigenetic indicators in the egg cells and early embryos of women with PCOS, suggesting that the risk could be transmitted even before implantation.
Though that does not mean that we can stop PCOS today, knowledge of these processes provides the hope of targeted intervention during fertility treatments like IVF someday. But these results are early and from lab-produced embryos. Additional studies—particularly in animal models and ultimately in the clinic—will be needed before these findings can be used to prevent PCOS in people. Nevertheless, the research is an encouraging step toward ending the cycle of PCOS from one generation to the next.
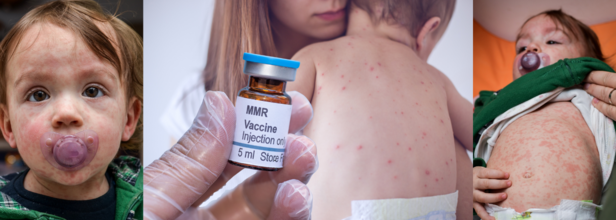
Measles Death In Liverpool Highlights Vaccine Urgency For Children: Here's What Parents Need To know
Credits: Canva
Health officials have urged parents to get their kids vaccinated in light of the measles outbreak, especially after a child at Alder Hey Children's Hospital in Liverpool died from the disease.
The city has experienced a surge in cases among young people. The hospital has also warned parents that there has been a spike in infections due to falling rates of uptake of the measles, mumps and rubella (MMR) vaccine.
What Is The MMR Vaccine?
As per the Centers for Disease Control and Prevention (CDC), the best way to protect against measles is to get the measles, mumps, and rubella (MMR) vaccine. For children, the measles, mumps, rubella, and varicella (MMRV) vaccine is prescribed, it also protects against chicken pox.
The National Health Service, UK, (NHS), notes that measles, mumps and rubella, also known as German measles spread easily between people and can lead to serious problems including meningitis, blindness and hearing loss.
NHS notes that if you are pregnant, getting measles can cause premature birth, miscarriage or even still birth. Rubella can also cause serious problems for your baby such as damage to their sight and hearing.
Also Read: Why Wimbledon Winner Jannik Sinner Was Banned: The Clostebol Drug Test Controversy Explained
NHS notes that with just two doses of MMR vaccine, you can get a long-term protection against the disease.
NHS notes that in the UK, there are two types of MMR vaccines available. You can review their ingredients in the official patient information leaflets:
- MMR VaxPro
- Priorix
MMR VaxPro contains a small amount of gelatine derived from pigs (porcine gelatine). If you'd prefer your child to receive the Priorix vaccine instead, speak to the healthcare professional administering the vaccine.
Who Should Have The MMR Vaccine?
The MMR vaccine is recommended for all babies and young children, but older children and adults can have it if they were not vaccinated when they were younger, notes NHS, UK.
Babies and young children are given 2 doses of MMR vaccine, which are also part of the NHS vaccination schedule. Children are given dose at 1 year old and 3 years 4 month old age. Babies between 6 and 12 months may have an extra dose of the MMR vaccine, especially if they are travelling abroad to an area with a lot of measles, they have been in close contact with someone with measles, and if there is a measles outbreak.
For older children and adults, notes NHS, the MMR vaccine can be administered at any age. However, it is important that you speak to the general practitioner (GP) about getting vaccinated in case you did not get the vaccine as a child or if you only had 1 dose.
Also Read: Damar Hamlin Is Back For 2025 NFL Season, But His Cardiac Arrest Has Changed The Course Of The Game
Who Cannot Have The MMR Vaccine?
Most people who need the MMR vaccine can safely receive it.
However, because it’s a live vaccine—meaning it contains weakened forms of the measles, mumps, and rubella viruses—it may not be suitable for everyone.
You should not get the MMR vaccine if:
- you are pregnant
- you have a weakened immune system due to a medical condition or medications that suppress immunity
- you’ve had a serious allergic reaction (anaphylaxis) to any ingredient in the vaccine, such as gelatine or neomycin
If you're feeling unwell: You can still get the MMR vaccine if you're mildly unwell, as long as you don’t have a high temperature.
If you have a fever or feel very unwell, it’s best to wait until you recover before getting vaccinated and ask your doctor.
© 2024 Bennett, Coleman & Company Limited

