- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
You Don’t Have To Smoke To Get Lung Cancer—Air Pollution May Be Just As Dangerous

Credits: Canva
For decades, lung cancer has been synonymous with smoking. But the data is shifting—and fast. Today, 10–20% of lung cancer cases in the U.S. are found in people who have never smoked a single cigarette. A new large-scale international study has now unearthed some of the strongest evidence yet that air pollution may be a major culprit—and it’s leaving a genetic trail behind.
Researchers have discovered that fine particulate matter in polluted air, commonly from traffic, industrial emissions, and smog, is strongly associated with DNA mutations that are also found in smokers’ lung tumors. These mutations may be key drivers of lung cancer development in never-smokers.
The research, involving whole-genome sequencing of lung tumors from 871 never-smokers across 28 regions in four continents, found that individuals living in highly polluted environments had significantly more driver mutations—the kind that directly trigger cancer.
The investigators matched tumor samples with long-term air pollution exposure estimates, using ground and satellite data for fine particulate matter (PM2.5). They found that non-smokers exposed to high levels of pollution were nearly four times more likely to exhibit the SBS4 mutational signature—a genetic fingerprint commonly linked to tobacco smoke.
Additionally, they identified a 76% increase in a separate signature associated with accelerated biological aging. This is alarming, considering these were individuals with no direct tobacco exposure.
What makes this finding even more significant is that researchers discovered TP53 and EGFR mutations—both known to be aggressive lung cancer markers—more frequently in people living in polluted areas. These genetic changes are typically hallmarks of cancers in smokers.
This implies that air pollution could be triggering similar molecular pathways to those activated by cigarette smoke.
But there was a twist: a new mutational signature, SBS40a, was found in 28% of never-smokers but not in smokers. The origin of this marker remains unclear, but it suggests that pollution may not be the only hidden risk.
Rise of Environmental Carcinogens
The study adds to a growing body of evidence that air pollution is not just an irritant—it’s a carcinogen. Fine particles can be inhaled deep into the lungs, where they may damage DNA directly or trigger chronic inflammation that promotes tumor growth.
Even more surprising, another carcinogen showed up in the data: aristolochic acid, found in some traditional Chinese herbal remedies. This compound was associated with a distinct mutational signature in patients from Taiwan, hinting at a possible secondary environmental risk factor for lung cancer in never-smokers.
The rise of lung cancer in non-smokers is especially noticeable in East Asia, where rates remain disproportionately high—particularly among women. While genetic predisposition may play a role, this new evidence points clearly to environmental exposures as a key contributor.
And it’s not just an Asian problem. In Western countries, urban dwellers are also exposed to dangerously high levels of air pollution. The World Health Organization estimates that 99% of the world’s population breathes air that exceeds safe pollution thresholds. This means the risk is truly global.
The power of this new research lies in its use of whole-genome sequencing to link pollution to DNA changes. These mutational signatures act as a kind of molecular journal, recording every environmental insult a cell has endured.
The ability to map those changes and match them to known pollutants gives researchers a more precise way to trace cancer origins—not just infer them from epidemiological studies.
While this study can’t definitively prove causation, the strong correlation between pollution exposure and cancer-driving mutations makes it clear that dirty air is more than just a nuisance—it's a potential trigger for deadly disease.
Researchers acknowledge some limitations. Pollution estimates were regional, not personal, meaning it’s unclear how much exposure each participant had. Self-reported smoking histories can also be unreliable.
Still, the pattern is unmistakable. Air pollution behaves like a mutagen, leaving behind signatures that align with known cancer mechanisms. And it appears to affect never-smokers in a strikingly similar fashion to tobacco users—down to the very DNA damage.
This study raises serious public health questions: If environmental exposure to polluted air can cause DNA mutations tied to cancer, what safeguards are in place to protect those most vulnerable?
Governments and public health agencies may need to reconsider air quality regulations, urban zoning, and access to clean air—especially in densely populated cities where pollution levels remain dangerously high.
Healthcare systems might also need to adapt. Traditional lung cancer screenings focus on long-time smokers, but this research could shift how we think about early detection in non-smokers, especially those living in high-risk environments.
Lung cancer has long been viewed through the lens of personal responsibility: if you smoked, you knew the risks. But this research changes that narrative. The air we breathe—something no one can fully avoid—is now emerging as a significant threat.
For non-smokers around the world, especially women and urban residents, this is a wake-up call. Your lungs may be at risk not because of personal choices, but because of public ones—decisions about pollution control, urban planning, and clean energy.
The future of lung cancer prevention may lie not just in quitting cigarettes, but in cleaning up the air we all share.
Soccer Player 'Choclo' Suffered A Type 3A Muscle Injury Causing His Absence From Match Against Delfín
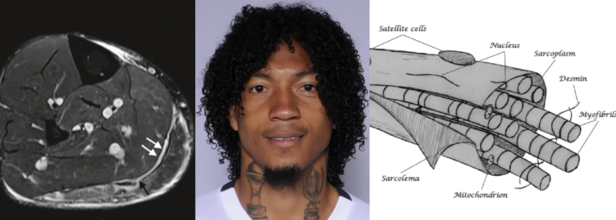
Source and in photos L to R: Minor, structural muscle injury (type 3a), source: SEMS Journal; player Choclo (source: LDU Quito); diagram of the muscle fiber, source: SEMS Journal
Liga Deportiva Univerrsitaria (LDU) has confirmed a new injury to its roster this Sunday. Defender José "Choclo" Quintero has a grade 3A muscle injury. This has been confirmed by team's official statement which has come from the club's medical department.
The player is currently undergoing rehabilitation and will begin strength training and physical therapy sessions. The official statement says that "Choclo" will have a reasonable amount of time before he returns to the field. He has so far played 19 matches and his return will depend on his progress.
A List Of Injuries
He had earlier suffered a skull fracture from August 30 2021 to October 4 2021 and as a result had missed three games in the season 21/22. Previously too in the season 16/17, he was in rehabilitation for 173 days due to a knew medial ligament tear, as a result of which he missed 31 games.
What Is A 3A Muscle Injury?
As per the 2014 study published in Translational Medicine @ UniSa, Official Journal of the Medical School of the University of Salerno, a 3A muscle injury is part of the structural injuries, which are divided into 3 sub-groups as per the "entity of the lesion within the muscle".
A type 3A lesion is a minor partial lesion, which involves one or more primary fascicles within a secondary bundle.
Symptoms
It usually is characterized by sharp pain, especially during a specific movement. The pain is, however, localized, and is easy to "appreciate on palpation and, at times, preceded by a snap sensation. On palpitation, it is not possible to detect the structural defect as it is too small and the contraction against manual resistance is painful," notes the study.
Diagnosis
Another study from 2016, published in the journal Joints, a 3A involves a minor partial muscle tear with small maximum diameter and could be detected through MRI that shows fiber disruption.
A 2019 study published in the journal Sport and Exercise Medicine Switzerland, notes that Type 3A are of less than 5mm in size. Thus, it includes 1-5 primary muscle bundles. The study also notes that such injuries usually heal without defect, however, injury to the perimysium and the aponeurosis is found in type 3b injuries (> 5 mm), which is the cause for intermuscular hematoma formation.
What Are The Common Muscle Injuries Among Football Players?
Type 3B injuries are moderate partial lesion, which involves at least a secondary bundle, with less than 50% of breakage surface. A Type 4 lesion is a sub-total tear with more than 50% of breakage surface or even a complete tear of the muscle. It also involves the muscle belly or the musculotendinous junction.
While these comprise structural injuries, Type 1 and Type 2 comprise the non-structural injuries.
It is the non-structural injuries, which are the most common and accounts for 70% of all muscle injuries in football players, mentions the 2014 study.
While the lesion may not be readily recognized in these injuries, they cause more than 50% of days of absence away from sport matches and training, the study notes. When they are ignored, they could turn into structural injuries.
Non-structural muscle injuries are classified into different types based on their underlying causes. Here's a breakdown of common types and what triggers them:
Type 1A Injury
These injuries are often the result of fatigue or sudden changes in training routines, running surfaces, or high-intensity activities. The muscle strain occurs due to the body not being fully adapted to new or excessive demands.
Type 1B Injury
This type of injury is linked to prolonged and excessive eccentric muscle contractions — when the muscle lengthens while under tension. This repeated strain can lead to overuse and damage.
Type 2A Injury
Type 2A injuries are typically associated with spinal issues. Often misdiagnosed, they stem from minor intervertebral disorders that irritate spinal nerves. This nerve irritation disrupts the normal control of muscle tone in specific “target” muscles. In such cases, treating the underlying spinal disorder becomes the main focus of care.
Type 2B Injury
These injuries are caused by a breakdown in the balance of the neuro-musculoskeletal system, particularly the mutual inhibition mechanism controlled by muscle spindles. When this balance is disrupted, the regulation of muscle tone suffers. Specifically, when the inhibition of agonist muscles is reduced, those muscles may become overly contracted to compensate — leading to dysfunction and injury.
Scientists Have Developed An AI Model To Help Predict Sudden Cardiac Risks 'Most Doctors Can't See'
Credits: Canva
Every year, thousands of seemingly healthy people—often young, active, and without obvious warning signs—die suddenly due to cardiac arrest. For decades, doctors have struggled to reliably identify which patients with heart conditions are at high risk and who might be unnecessarily undergoing invasive interventions. That may be about to change.
In a breakthrough that could transform how we predict—and prevent—sudden cardiac death, scientists at Johns Hopkins University have developed an artificial intelligence model that vastly outperforms current clinical standards in identifying people most at risk. Their new system, known as MAARS (Multimodal AI for Arrhythmia Risk Stratification), not only forecasts risk with up to 93% accuracy in vulnerable age groups, but also explains why someone is high risk—something most algorithms fail to do.
The focus of the study is hypertrophic cardiomyopathy (HCM), one of the most common inherited heart conditions. It affects around 1 in 200 to 500 people globally and is a leading cause of sudden cardiac death in athletes and young adults. While most individuals with HCM live normal lives, a subset is at high risk for lethal arrhythmias—heart rhythm disturbances that can cause the heart to stop without warning. And here’s the catch: right now, doctors only have a 50-50 shot at predicting who will be affected.
“Currently we have patients dying in the prime of their life because they aren’t protected,” said Dr. Natalia Trayanova, senior author of the study and a leading figure in AI cardiology research. “And others are putting up with defibrillators for the rest of their lives with no benefit.”
Trayanova is referring to implantable cardioverter defibrillators (ICDs)—tiny devices inserted into the chest that deliver electric shocks to correct abnormal heart rhythms. They save lives in the right patients but come with physical, emotional, and financial burdens when used unnecessarily.
The need for a more precise, personalized tool has never been greater.
What the AI Model Does Differently?
Published in Nature Cardiovascular Research, the new model represents a significant departure from traditional clinical guidelines used across the US and Europe.
MAARS doesn’t rely on a single data source. Instead, it analyzes a multimodal spectrum of information—ranging from electronic health records and patient histories to contrast-enhanced cardiac MRI images that reveal scarring, or fibrosis, within the heart.
Scarring is a key factor in determining sudden death risk in HCM. But interpreting these raw images is extremely challenging for even seasoned cardiologists. That’s where AI has the edge.
“People have not used deep learning on those images,” Trayanova explained. “We are able to extract this hidden information in the images that is not usually accounted for.”
The AI essentially spots dangerous patterns in the heart’s scar tissue that the human eye—and even conventional software—can’t see.
How Accurate Is It?
In clinical tests involving real-world patients from Johns Hopkins Hospital and Sanger Heart & Vascular Institute in North Carolina, the results were staggering:
- Traditional clinical guidelines correctly predicted sudden cardiac death risk only 50% of the time.
- The MAARS model achieved an overall accuracy of 89%.
- In patients aged 40 to 60—a group particularly vulnerable to undetected risk—the model was 93% accurate.
What makes this even more valuable is its ability to provide explanations. The system doesn't just say "this patient is high risk"—it breaks down the why, giving cardiologists critical information to tailor treatment plans.
“This significantly enhances our ability to predict those at highest risk compared to our current algorithms,” said co-author Dr. Jonathan Crispin, a Johns Hopkins cardiologist. “It has the power to transform clinical care.”
Why This Could Be Transformative?
MAARS isn't the first AI model from Trayanova’s lab. In 2022, her team built another tool that provided survival predictions for patients with prior heart attacks, known as infarcts. But this latest model breaks new ground by tackling one of the most elusive forms of cardiac risk—arrhythmias caused by scarring in inherited heart conditions. The potential benefits are wide-ranging:
- Lives saved by identifying at-risk patients who might otherwise be missed.
- Better quality of life for patients who avoid unnecessary ICD implantation.
- More personalized treatment plans based on detailed, AI-generated insight.
Importantly, the model was trained and validated across diverse demographics, showing consistent performance regardless of age, gender, or ethnicity.
The researchers aren’t stopping here. They plan to expand MAARS to include other forms of arrhythmia-related heart diseases, such as cardiac sarcoidosis and arrhythmogenic right ventricular cardiomyopathy—conditions that also carry a high risk of sudden death but suffer from diagnostic ambiguity.
They’re also working to test the model in larger, more varied populations to move it closer to clinical adoption.
A New Era in Cardiology?
Artificial intelligence has long been hyped as the future of medicine. But MAARS is more than hype—it’s a working proof of concept that shows how deep learning can complement medical expertise, not replace it.
AI may soon become your cardiologist’s most powerful diagnostic tool—one that sees what even the best-trained human eyes might miss. And when lives are on the line, that kind of clarity could mean everything.
1 In 8 Babies Born Premature In India, 17% Having Low Birth Weight— Health Survey Blames Air Pollution

Credits: Canva
India is struggling with alarmingly high levels of premature deliveries and low birth weights in infants, and the cause could be one of the nation's most pressing environmental threats—air pollution.
As per India's National Family Health Survey-5 (2019–21), about 13% of infants were born pre-term and 17% were low birth weight, with the research identifying airborne fine particulate matter or PM2.5 as a key driver of these negative birth outcomes. The results, published in PLoS Global Public Health, are the outcome of cross-institutional collaboration between Indian and global research centers. By uniting large-scale health survey data with air quality remote sensing, the scientists have mapped not only the extent of the problem but also its underlying environmental causes.
The research, conducted in association with India's leading scientific institutions such as IIT Delhi, International Institute for Population Sciences (Mumbai), and collaborating UK and Irish partners, merged public health information with high-resolution satellite images to evaluate the impact of air pollution on pregnancies. With sophisticated spatial modeling and statistical analysis, researchers found that increased exposure to PM2.5 during pregnancy was associated with a 70% greater risk of preterm birth and a 40% greater chance of low birth weight.
This is a major public health issue, with both outcomes having long-term health consequences for infants, from cognitive disabilities to chronic conditions such as diabetes and heart disease in adult life.
What Connects Air Pollution with Preterm Births?
At the center of the crisis is fine particulate matter (PM2.5)—small airborne particles smaller than 2.5 microns in diameter, emitted primarily from the combustion of fossil fuels and biomass. The particles are tiny enough to reach deep into the lungs and into the bloodstream, and they threaten the health of mothers and fetuses equally.
The research determined that higher exposure to PM2.5 during pregnancy was linked with a 40% increased likelihood of low birth weight and a 70% increased risk of preterm delivery. The rise of 10 microgram per cubic meter (μg/m³) in PM2.5 exposure was correlated with a 5% increase in the prevalence of low birth weight and a 12% increase in preterm delivery.
These results are consistent with global evidence, including a recent meta-analysis, which reported a similar dose-response pattern between exposure to PM2.5 and adverse birth outcomes globally. Exposure to other PM2.5 components—black carbon, nitrates, and sulfates—has also been associated with spontaneous preterm birth, especially in the second trimester.
The research identified glaring regional inequities. States in the higher Gangetic plains of North India, including Punjab, Delhi, Uttar Pradesh, Haryana, and Bihar, demonstrated the greatest PM2.5 levels. These states also had the greatest rates of premature births and low birth-weight babies. For example, Himachal Pradesh reported a whopping 39% premature birth rate, followed by Uttarakhand (27%), Rajasthan (18%), and Delhi (17%).
Conversely, the northeastern states of Mizoram, Manipur, and Tripura did notably better, with reduced air pollution rates and associated improved outcomes at birth. The results indicate the imperative for focused policy action in northern India, where urbanization, agricultural burning, and the use of fossil fuels are pushing perilously bad air quality.
Can Climate Change Is Add to the Crisis?
Aside from air pollution, other climate factors like temperature and changes in rainfall patterns also affected pregnancy outcomes, according to the Indian study. The study observes that intense heat waves, irregular monsoons, and water shortages—characteristics of the climate emergency—can directly affect the health of the mother and fetus.
With this convergence of environmental and reproductive health, specialists are increasingly advocating for the incorporation of climate adaptation measures in public health planning. Localized heat action plans, improved water management systems, and effective risk communication systems are among these.
How Air Pollution Harms Pregnancies?
The journey from contaminated air to poor birth outcomes is both subtle and direct. PM2.5 and its components have the ability to penetrate the placental barrier, leading to inflammation and oxidative stress in the placental tissue. This inflammation is directly related to preterm labor, low birth weight, and even neurodevelopmental delay in children.
Black carbon, one of the dominant fractions of PM2.5, has been found to interfere with fetal development and raise the risk of preeclampsia and preterm rupture of membranes, further putting mother and child at risk. The additive effect is increased odds of babies being born too early or too light, with health consequences for life.
India's National Clean Air Programme (NCAP), initiated in 2019, plans to lower PM levels by 20–30% in 122 non-attainment cities by 2024. It's good that it is a move towards improvement, but according to researchers, efforts need to be scaled up and enforced more strictly, particularly in the northern belt where pollution levels are still critically high.
The researchers of the study also suggest greater public health outreach, including education campaigns for pregnant women, frontline health workers, and policymakers. Education about air quality monitoring, prenatal care access, and simple measures to avoid exposure are crucially necessary.
How Pregnant Women Can Protect Themselves?
Although systemic change is necessary, there are also measures individuals—particularly pregnant women—can take to lower their risk:
- Monitor local air quality and limit outdoor activities when pollution levels are high.
- Use air purifiers and maintain clean indoor environments.
- Avoid heavily trafficked roads and industrial areas whenever possible.
- Eat a diet rich in antioxidants, which may help counteract some of the harmful effects of air pollutants.
Yet, with air pollution still on the increase, such individual precautions need to be supplemented by strong public health and policy measures so that meaningful protection for both mothers and infants is guaranteed.
This research reinforces a growing body of international evidence that links air pollution to reproductive and neonatal health risks. According to the World Health Organization, over 90% of the global population breathes unsafe outdoor air, and half are exposed to indoor pollution from traditional cooking methods involving coal, dung, or wood.
Worldwide, 15 million babies are born prematurely each year, and so preterm birth is the predominant cause of neonatal deaths. By which standards is the Indian study a local health concern—it's a global warning for health.
© 2024 Bennett, Coleman & Company Limited

