- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
AI Creates Antibiotics That Could Defeat Drug-Resistant Bacterial Infections
Credits: Health and me
The global public health community faces a growing crisis as antimicrobial resistance (AMR) continues to make common antibiotics useless, leading to more than one million deaths annually. To address this, scientists at the Massachusetts Institute of Technology (MIT) have used artificial intelligence to create two new antibiotics, NG1 and DN1, which have been found to be very effective against extremely resistant bacterial pathogens, such as Neisseria gonorrhoeae (gonorrhoea) and methicillin-resistant Staphylococcus aureus (MRSA).
This breakthrough is a significant leap towards the battle against drug-resistant infections, giving hope to patients and clinicians worldwide.
Traditional methods of antibiotic development depend extensively on screening current chemical libraries for compounds capable of inhibiting bacterial growth. While this method has been successful in the past, it has its limitations in range and velocity, especially for emerging fast-evolving drug-resistant strains.
MIT researchers employed generative artificial intelligence (AI) to explore previously inaccessible chemical spaces. With two different generative AI methods—chemically reasonable mutations (CReM) and fragment-based variational autoencoder (F-VAE)—the scientists engineered more than 36 million theoretical compounds. The compounds were computationally tested for antimicrobial activity, structural originality, and synthesizability.
MIT's Termeer Professor of Medical Engineering and Science, Dr. James Collins, described: "Our research demonstrates the potential of AI from a drug design perspective. It allows us to tap into enormous chemical spaces that were inaccessible to us before, speeding up the discovery of antibiotics with completely new mechanisms of action."
The computer-aided design process screened the enormous number of molecules down to a handful of potential candidates for laboratory synthesis. In the case of N. gonorrhoeae, the researchers used a fragment-based strategy, discovering a lead chemical fragment, F1, and creating millions of derivative molecules. Following computational screening and synthesis, a top compound, NG1, was highly effective. Tests in the laboratory and mouse models verified its capability to suppress LptA, a protein required for bacterial membrane synthesis.
For S. aureus, an open-ended design strategy generated 29 million compounds, 22 of which were synthesized. Six candidates exhibited high antibacterial activity in vitro, with DN1 showing the ability to kill MRSA in a mouse skin infection model.
The import of these findings is not simply in their activity but also in their unique mechanisms. By acting on bacterial membranes in manners distinct from current antibiotics, NG1 and DN1 diminish the risk of accelerated resistance emergence, an important challenge of contemporary antimicrobial treatment.
How Does This Discovery Contribute to the Global AMR Crisis?
Antimicrobial resistance poses a mounting threat to public health. Bacteria adapt quickly, and traditional antibiotics struggle to keep up, with treatment-resistant infections becoming more difficult to treat. Gonorrhoea and MRSA are just two high-profile examples, with the former increasingly resistant to first-line treatments and the latter causing debilitating hospital-acquired infections.
By introducing AI-designed antibiotics, researchers hope to stay ahead of bacterial evolution. These drugs could form the foundation of a new generation of antimicrobials, effective even against strains that have outsmarted traditional therapies.
Implications Beyond Gonorrhoea and MRSA
While NG1 and DN1 are only at the outset of development and need to undergo major clinical testing before being available for humans, the methodology itself is a revolution in drug discovery. The same strategy using AI could be used to create antibiotics against other bacterial pathogens, and potentially solve many resistant infections.
The approach of the MIT team also points to the wider potential of computer-aided drug design, allowing researchers to explore chemical spaces too vast for regular lab screening. This would speed up the discovery of drugs not just for bacterial disease but also for viral and fungal pathogens.
What Are The Challenges?
Although promising, AI-generated antibiotics are not yet clinically deployable. NG1 and DN1 will need to be subjected to extensive testing to determine safety, effectiveness, and lack of side effects in humans. Additionally, regulatory approval procedures for new compounds can take years, with meticulous examination at each step.
Another aspect to consider is the constant war with bacteria. Although NG1 and DN1 use new mechanisms, bacteria can potentially learn countermeasures. Ongoing surveillance and repeated cycles of drug design will be necessary to keep the advantage.
What Is The Role of AI in Future Medicine?
This advance highlights the revolutionary promise of AI in medicine. Aside from antibiotics, AI is being used more and more to discover drug candidates for cancer, neurological diseases, and metabolic disease. Through molecular interactions simulated and biological activity predicted, AI can decrease by vast orders of magnitude the time and expense of taking new drugs from idea to clinical trials.
As Dr. Collins said, "AI enables us to push the boundaries of drug discovery, opening up possibilities that were unimaginable before. This is only the start of a new frontier in antimicrobial therapy and precision medicine."
Development of NG1 and DN1 is especially apt given the growing travel and globalization, which advance the speed at which drug-resistant bacteria can spread. Gonorrhoea, for example, has demonstrated escalating resistance across a number of countries, making standard treatment regimens difficult. MRSA continues to be a major cause of hospital infections, putting healthcare systems under pressure globally.
Breakthroughs such as AI-designed antibiotics may be central to preventing future crises, in addition to vaccination campaigns, hygiene practices, and judicious antibiotic use.
The MIT researchers' discovery of AI-designed antibiotics NG1 and DN1 is a fantastic milestone in the war on antimicrobial resistance. Through the use of computational strategies to scan large chemical spaces, scientists have created compounds with new mechanisms that can target drug-resistant gonorrhoea and MRSA.
Frida Baby Thermometer Under Scrutiny After Brand Sexualizes Its Packaging
Credits: Canva (Representational)
Frida Baby thermometer is facing severe backlash after parents criticized the company's marketing language on its packaging. The parents claimed that the company cracked sexual jokes on using baby thermometers and that it crossed a line for a product that was designed for infants.
The controversy gained more traction this week after a social media user posted about it on X with photos stating that the marketing relies on sexual jokes.
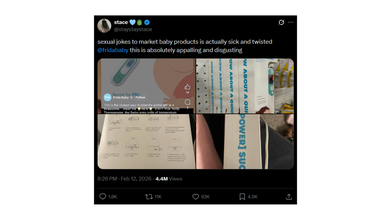
An X user @staystaystace wrote: "sexual jokes to market baby products is actually sick and twisted @fridababy this is absolutely appalling and disgusting".
Frida Baby Thermometer: What Was The Problem With Marketing

The photos include a screenshot of the thermometer packaging on which the graphics are pointed out to be problematic and inappropriate by the parents. Furthermore, the caption on the official account of Frida Baby social media reads: 'This is the closest your husband's gonna get to a threesome...'
In another photo of Frida Baby 3-in-1 Eat, Forehead and Touchless Thermometer, the marketing phrase reads: 'How About A Quickie?'
Another photo on steps for using the humidifier, the caption on the packaging reads: 'I Get Turned On Easily'. While a fourth photo reads: 'I'm A [Powerful] Sucker'.
Parents claim that the brand has used sexual market phrases found similarly on self care toys and massagers for products which are made for infants.
Read: Six-year-old Child Dies Of Medical Negligence During MRI At Greater Noida Imaging Centre
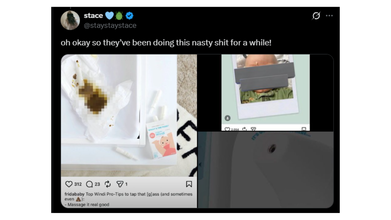
Amid this, older content from 2020, including deleted social media posts resurfaced featuring a baby with visible nose discharge and the caption read: 'What Happens When You Pull Out Too Early #nosefrida #dontmove'.
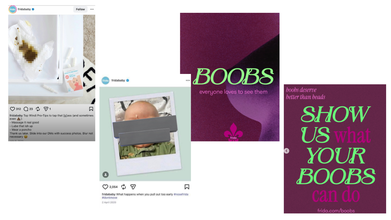
More recently, Frida Baby again drew attention for an Instagram post that centered on breastfeeding. The carousel post includes slides with statements like: 'Boobs, everyone loves to see them', followed by commentary on how breasts are widely accepted in pop culture, but criticized in the context of feeding a child. The final slide reads: 'Show Us What Your Boobs Can Do', which directs readers to company's website. A user on the Instagram post commented: "Hey so why do you sexualize your products??"
Another photo that resurfaced from 2021 reads: 'Top Windi Pro-Tips to tap that [g]ass (and sometimes even [poop emoji]):
- - Massage it real good
- - Lube that ish up
- - Wear a poncho
However, there were some parents who also defended the brand's tone and argued that humor is clearly aimed at adults who are navigated through the realities of parenting.
Frida Baby Thermometer: How Did The Brand React?
In an emailed statement, as reported by Complex, the spokesperson for the brand said:
From the very beginning, Frida has used humor to talk about the real, raw, and messy parts of parenting that too often go unspoken. We do this because parenting can be isolating and overwhelming, and sometimes a moment of levity is what makes a hard experience feel human, shared, and survivable.
Our products are designed for babies, but our voice has always been written for the adults caring for them. Our intention has consistently been to make awkward and difficult experiences feel lighter, more honest, and less isolating for parents.
That said, humor is personal. What’s funny to one parent can feel like too much to another. We’re never trying to offend, push boundaries for shock value, or make anyone uncomfortable. Importantly, our tone is never separate from our product. The humor we use is always grounded in a specific feature, benefit, or innovation — a reflection of the real problem we are solving for families.
Frida was built to support families through some of the most vulnerable and transformative chapters of their lives. We stand firmly behind that mission. We will continue to show up with honesty, empathy, and courage.
With each decision we make, we will continue to evaluate how we express our voice so that our commitment to families is unmistakable and our tone always meets the moment.
Six-year-old Child Dies Of Medical Negligence During MRI At Greater Noida Imaging Centre
Credits: Canva
A six-year-old boy died after his health worsened during an MRI scan at a private Greater Noida diagnostic centre. His family alleged medical negligence and claimed that he was administered a wrong or heavy dose injection.
As per the boy's father, Prashant Kasana, his son was taken to the centre for some test and was given an injection before the MRI procedure. The family said that during the MRI scan, the child was administered a heavy dose. Due to which his condition worsened and he also lost consciousness.
The family also said that when they asked for information about the child's condition and his medical report, they were not given any satisfactory answers. They also claimed that the doctors gave another dose to the child. The child's condition did not improve and the family had to rush the child to another nearby private hospitals. This is where the doctors declared him dead.
Six-year-old Child Dies of Medical Negligence: What Happened?
After this incident, family members accused the staff of the KB Healthcare Centre, where the child was first taken for an MRI scan. Villagers and workers of the Bharatiya Kisan Union also reached the spot and staged a protest.
The state spokesperson of the Bharatiya Kisan Union, Pawan Khatana, stated that the child was brought to the centre at 10.30am, and was in normal condition. As per the spokesperson, doctors did not disclose the quantity of the dose administered to the child.
As per Khatana, even after half an hour, the child did not gain consciousness and when the doctors checked him again, he was unresponsive and cold. The family took to another private hospital where he was declared dead. Khatana also alleged that there are many such unauthorized screening and imaging centres operating in Greater Noida and demanded a thorough probe.
Six-year-old Child Dies Of Medical Negligence: What Are The Authorities Saying?
Police on reaching the spot received the information, while protesters demanded for a fair investigation and strict action against those responsible. The Station House Officer of Neta 2 police station said the child was a resident of Reelkha in Dankaur. He was brought to a private pathology lab in Sector P3 for an MRI scan. As per the officer, the doctor administered the child with anesthesia for an MRI. After this, the child's health started to deteriorate.
Police has sent the body for post-mortem after completing the necessary legal formalities.
Six-year-old Child Dies of Medical Negligence: How Much Anesthesia Is Safe For A Child During MRI?
As per the National Institutes of Health (NIH), US and a study by the Yeungnam University Journal of Medicine (YUJM), drugs for deep sedation or general anesthesia for pediatric MRI are:
(I) Chloral hydrate: a sedative hyptonic agent with no analgesic properties
The study notes that the recommended dose of chloral hydrate is 50 to 100 mg/kg, or up to a maximum of 2g. The success rate of chloral hydrate sedation for pediatric MRI varies from 78% to 100%.
The United Kingdom (UK) National Institute for Clinical Excellence (NICE) also recommends the use of oral chloral hydrate with a wide margin of safety in children under 15 kg.
The study also notes that children "may be encouraged to take at least clear fluids 2 hours before the procedure for successful sedation without breaking institutional fasting protocols for chloral hydrate sedation".
(II) Pentobarbital: a medium duration barbiturate that provides potent sedation with no analgesic property.
As per the study, this can be administered via an oral or intravenous or IV route. The oral dose is administered between 4 to 8mg per kg and IV dose of 2 to 3 mg per kg.
(III) Midazolam: it is a short-acting water soluble benzodiazepine that has anxiolytic, sedative, amanestiec, and muscle relaxant properties.
It is administered through various routes, but IV is preferred. When administered through IV, it is given at the dose of 0.1mg per kg.
Anesthetic agents include propofol and sevoflurane.
Note: This article is not a substitute for medical consultation or prescription. The information is based on reports and research articles available online for public.
Trump's Slurred Speech At Champion of Coal Event Sparks Health Concerns

(Credit - The White House/X)
President Donald Trump’s slurred speech at a recent event has sparked renewed concerns for his health. During a White House ceremony, where President Trump was being crowned the “Undisputed Champion of Coal”, his speech briefly slurred and he mispronounced the word, “undisputed.”
A video clip of him saying “And I'm proud to officially name the undithpuut... When did this come out, Mr speaker.” has been making rounds on social media platforms like X. Speculations about President Trump’s dementia and memory loss have been taking over the internet. People are also drawing between the supposed email mentioned in the Epstein files that also mentioned Trump’s supposed memory loss and the continued rumors about his dementia.
Also read: Epstein Files Raise Questions About Trump’s Memory Decline
Is Slurred Speech A Sign of Dementia?
According to Harvard Health, yes, slurred speech can be a sign of dementia, more specifically vascular dementia. While many people associate dementia primarily with memory loss seen in Alzheimer’s, vascular dementia has a different cause and set of symptoms.
Vascular dementia is the second most common form of the condition. It is caused by reduced blood flow to the brain, often due to cholesterol-clogged vessels or a series of "silent" mini-strokes.
Health Conditions That Can Cause Slurred Speech?
Harvard health also explains that slurred speech typically appears in two scenarios
Following a Major Stroke
A significant blockage can cause an abrupt mental shift, often paired with physical symptoms like paralysis or slurred speech.
As a Core Symptom
Because symptoms depend on which specific area of the brain is damaged, some individuals may experience slurred speech and confusion even if their memory remains relatively intact.
Also Read: Donald Trump Alzheimer’s Speculation Rises After Niece Notices Worrying Sign
Trump’s Dementia Speculations
With the influx in conversation surrounding the health of President Donald Trump, it is important to remember that these are not proven claims. On social media, people often point to these "fumbles" as evidence of the president’s cognitive decline or dementia. However, we must understand that the situation is much more complex than that
The White House maintains that the President is in "excellent overall health" and has even released MRI results to back that up. Trump himself often pushes back aggressively against the media for questioning his fitness.
While the public is quick to speculate, experts like neurologists and specialists in aging, say we should be careful. They argue it is impossible, and unprofessional, to diagnose someone just by watching them on TV.
Other Reasons Why Trump’s Health Made News
In 2025, public debate frequently centered on President Trump’s physical health, sparked by visible signs like hand bruising and swollen ankles.
Hand Bruising
Health doubts had been sparked when a bruise on Trump’s hand was highlighted. However, the White House clarified that the bruising on his right hand was a common side effect of daily Aspirin use for heart health, potentially worsened by frequent handshaking.
Swollen Ankles
Similarly, his swollen ankles during the ASEAN summit were attributed to Chronic Venous Insufficiency (CVI), a condition where leg veins struggle to pump blood back to the heart, in an official report from the White House.
MRI Scan
Official MRI results of President Trump also described his cardiovascular and abdominal health as "excellent,". This was shared by Dr. Sean Barbabella, the White House physician, who said that the scans were routine checkups for a 79-year-old and were exceptional for his age.
Cognitive Screening
Trump claimed he "aced" an IQ test, but experts identified it as the MoCA dementia screening. This basic 10-minute exam checks for cognitive impairment rather than measuring a person's general intelligence.
© 2024 Bennett, Coleman & Company Limited

