- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
- Web Stories
Jessie J Health Update: Hospitalized Weeks After Cancer Surgery Due To Lung Infection
Credits: Canva
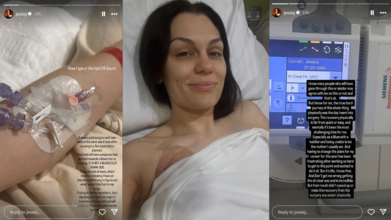
Six weeks after undergoing breast cancer surgery, singer Jessie J revealed that she was unexpectedly hospitalized again, this time due to a suspected lung issue. The 37-year-old artist, known for her chart-topping hits like Price Tag and Domino, shared that she returned to the same hospital ward where she had recovered post-surgery.
In an emotional update on Instagram, she wrote: “Six weeks post-surgery and I was back in the same ward I was after my surgery. Not expected or planned.”
Doctors initially suspected a blood clot in her lung due to concerning symptoms, but further tests ruled that out. Instead, they discovered she had an infection and a small amount of fluid in her lungs. The exact source of the infection is still being investigated.
Despite ongoing symptoms and difficulty breathing, Jessie J said she chose to discharge herself from the hospital: “I hate being in hospital and will continue the investigation as an outpatient.”
What Happened To Her?
The singer said she was experiencing troubling symptoms that hinted at a pulmonary embolism. “I had and still have symptoms that pointed towards a blood clot on the lung. IT IS NOT A BLOOD CLOT THANK GOD,” she shared. “They ran a lot of tests, which ended up showing I have an infection (still trying to figure out what) and a little fluid on my lungs.”
In the post, she also included a photo showing a cannula inserted in her arm, highlighting the seriousness of her condition and the fatigue that comes with recovery.
Adjusting to a Slower Recovery
The singer underwent a mastectomy and breast reconstruction surgery after being diagnosed with early-stage breast cancer earlier this year. In July, she had celebrated the news that there was no sign of cancer spread.
However, her latest update highlights the emotional and physical toll of recovery. “The recovery physically is far from quick or easy,” she said. “And mentally, it’s been the most challenging time for me, especially as a mum with a toddler and being unable to be the mother I usually am.”
Jessie J welcomed her son, Sky Safir Cornish Colman, in 2023 after previously experiencing a miscarriage in 2021. She has been open about the complexities of motherhood alongside serious health challenges.
Impact on Career and Mental Health
The star also spoke about the career setbacks caused by her ongoing health issues. “Having to change the plans for my career for this year has been frustrating after working so hard to get to this point and excited to do it all. But it’s life. I know that,” she admitted.
She said that while getting the all-clear from cancer was “incredible,” it didn’t make the physical healing from surgery any easier. The reality, she added, is that recovery takes time and patience.
A Reminder to Slow Down
Known for her high-energy performances and fierce work ethic, Jessie J described the shift in pace as a tough adjustment. “As an ADHD Aries, fire-breathing dragon T-Rex, I can do it myself, I’m always ok woman. That slow pace has been a hard reality to accept to be honest,” she wrote.
Despite the frustrations, she acknowledged that this health scare served as a wake-up call. “This isn’t a speedy recovery, and it isn’t meant to be,” she said. “It’s a reminder to myself to slow down, even though I felt I already was.”
Jessie J's History of Health Battles
This isn’t Jessie J’s first experience with serious health issues. Diagnosed with a heart condition at the age of eight, she suffered a minor stroke at 18 and temporarily lost hearing in 2020. Yet despite her setbacks, the Brit Award-winning artist has remained candid about her health journey and determined to keep going.
Tylenol And Autism: Trump, RFK Jr., And Officials To Link The Two In An Announcement Soon

Credits: Tylenol and AP
Autism and President Donald Trump, at this moment goes hand in hand, as ever since Trump's administration, with the help of his handpicked Health Secretary RFK Jr. decided to conduct a study to examine the potential link between vaccines and autism. The Centers for Disease Control and Prevention (CDC) had been told to study for the link between the two, a theory very well backed by President Trump and RFK Jr.
Up new is the recent plan to announce by the President on Monday that using Tylenol, a brand of medicine, used for reducing pain or fever, during pregnancy could increase the risk of autism. Two senior administration officials who have confirmed this news to POLITICO also said that advisory will be issued for pregnant women to only use generic acetaminophen for high fevers.
The officials have also told the media outlet that Trump may also highlight the benefit of leucovorin, which is a cancer and anemia drug as a potential therapy for people with autism.
"Autism Is Totally Out Of Control"
Not too long ago, on Friday, Trump himself said that he will soon be making a big announcement on the neurological condition. "Autism is totally out of control. I think we, maybe have a reason why," he told the reporters.
White House spokesperson Kush Desai, in a statement on Sunday said that the "announcement will make historic progress" in addressing rising autism rates. As per CDC's data, about 1 in 31 children aged 8 years has been identified with autism spectrum disorder (ASD). Among them 1 in 6 children aged 3 to 17 years were diagnosed with a developmental disability, during the period study of 2009-2017. These also included attention-deficit/hyperactivity disorder (ADHD)., blindness, and cerebral palsy.
The CDC also reported this spring that 1 in 31 American 8-year-olds was diagnosed with the condition in 2022, compared with 1 in 150 in 2000.
Why Autism? To Find The Answer Is HHS' Major Priority
Health Secretary Robert F Kennedy Jr. also promised earlier this year to have "some" answers to the question of why cases are increasing. This has been made HHS' major priority.
As per Kennedy, there are environmental factors too, though there is enough evidence to counter his argument. For him, vaccines are involved.
Read More: CDC Plans Vaccine-Autism Study Despite Scientific Consensus
Coming Back To Tylenol
Acetaminophen, sold under the brand name Tylenol, is the most widely used medication for pain and fever relief among pregnant women, who are generally advised to avoid ibuprofen (Advil) because of its link to miscarriage and birth defects.
According to a senior administration official, RFK Jr. and other top health leaders are expected to take part in an upcoming announcement. However, officials remain divided on how to address the sensitive issue of autism. Kennedy himself has expressed concern about a possible link between acetaminophen use during pregnancy and autism but has been cautious about issuing a public warning.
The administration is simultaneously preparing a broader autism initiative. About a dozen working groups are set to investigate roughly 30 possible causes of the condition. A supporting literature review, still underway, will not be released ahead of Monday’s announcement.
Earlier this year, Kennedy pledged to identify the cause of autism by September, a timeline many researchers considered unrealistic. The National Institutes of Health, tasked with leading the effort, has already tempered expectations, saying new grant funding will launch this fall, with meaningful updates not expected until next year.
This month, The Wall Street Journal reported that the Department of Health and Human Services (HHS) intended to connect autism risk to Tylenol use in pregnancy, as well as to folate deficiencies in women, with leucovorin being floated as a possible therapy. But officials later confirmed that no such report currently exists, as reported by POLITICO.
Meanwhile, a review published last month in BMC Environmental Health by Harvard’s T.H. Chan School of Public Health dean Dr. Andrea Baccarelli and colleagues linked acetaminophen use to autism and urged caution. The authors recommended that pregnant women take the drug only when necessary, at the lowest effective dose and for the shortest duration, but stopped short of advising a complete ban.
Industry leaders are pushing back. According to The Wall Street Journal, Kirk Perry, interim CEO of Tylenol manufacturer Kenvue, privately urged Kennedy not to name Tylenol as a cause. Kenvue, spun off from Johnson & Johnson in 2023, considers Tylenol a flagship brand.
In a statement to POLITICO, a Kenvue spokesperson rejected any claims of a link. “Over a decade of rigorous research, endorsed by leading medical professionals and global health regulators, confirms there is no credible evidence connecting acetaminophen to autism,” the company said. It added that discouraging Tylenol use could leave women facing “dangerous choices” between untreated pain, which itself can harm mothers and babies, and riskier alternatives.
Major medical groups continue to support acetaminophen use in pregnancy. The American College of Obstetricians and Gynecologists maintains that there is “no clear evidence” tying appropriate use to fetal developmental problems and warns against leaving pain or fever untreated.
So far, studies on acetaminophen and autism have largely been observational, pointing to associations but not proving causation.
World Alzheimer's Day 2025: Theme, Origin, And Significance

Credits: Canva
World Alzheimer’s Day, observed annually on September 21, once again drew global attention to the rising burden of Alzheimer’s disease and the urgent need for collective action. This year, the 2025 theme emphasizes the importance of early awareness, timely diagnosis, and stronger community support systems to help patients and families cope with the challenges of dementia.
Breaking Stigma Around Dementia
Alzheimer’s disease is the leading cause of dementia worldwide. It is a progressive neurological disorder that impairs memory, thinking, and the ability to perform everyday tasks. Over time, it not only affects individuals but also places a heavy emotional and physical toll on families and caregivers.
The stigma around dementia often prevents people from seeking medical help in the early stages. Symptoms such as forgetfulness, disorientation, or difficulty handling daily tasks are too often dismissed as “normal aging.” Experts, however, warn against such assumptions.
“Alzheimer’s is not simply about age-related memory loss, it is a serious neurological condition that needs to be identified early,” noted Dr. Pavan Pai, Consultant Interventional Neurologist at Wockhardt Hospitals, Mira Road, as reported in the Free Press Journal. “Recognizing these signs early allows us to intervene in time, slow down progression through therapies, and prepare caregivers to provide better support.”
By focusing on awareness and early detection, this year’s campaign aims to reduce stigma and empower families to act before the disease advances.
The Global Burden of Alzheimer’s
More than 55 million people worldwide currently live with dementia, and the number is projected to rise sharply with aging populations. In India and other developing countries, cases are steadily increasing, adding to public health challenges.
Alzheimer’s is not only a medical condition but also a social issue, as it disrupts family structures and places immense demands on caregivers. Recognizing this, World Alzheimer’s Day encourages governments, organizations, and communities to work together to improve patient care, caregiver support, and social acceptance.
Origin and History of World Alzheimer’s Day
The first World Alzheimer’s Day was launched on September 21, 1994 by Alzheimer’s Disease International (ADI), a federation of Alzheimer associations across the globe. It was timed to mark the 10th anniversary of the organization.
Over the years, the campaign grew in scale and visibility. In 2012, ADI expanded the initiative into World Alzheimer’s Month, transforming September into a full month of awareness activities. Since then, the annual campaigns have included educational seminars, fundraising walks, memory cafés, and advocacy programs.
These initiatives not only increase understanding of dementia but also provide platforms for patients and caregivers to share experiences and seek community support.
Why World Alzheimer’s Day Matters
World Alzheimer’s Day is not just about raising awareness but also about inspiring collective action. The day underlines several critical areas:
Early diagnosis: Detecting Alzheimer’s in its initial stages improves chances of slowing progression through therapies and lifestyle interventions.
Access to care: Patients need medical, social, and emotional support systems to live with dignity.
Reducing stigma: Myths and misconceptions must be challenged to encourage acceptance and compassion.
Research and innovation: Ongoing scientific research is essential to find better treatments and, eventually, a cure.
Around the world, communities organize activities such as memory walks, caregiver workshops, and awareness drives. These efforts encourage societies to become more dementia-friendly, ensuring patients and families do not feel isolated.
Moving Forward With Hope
As the global population ages, Alzheimer’s and other dementias will continue to challenge healthcare systems, families, and communities. World Alzheimer’s Day 2025 serves as a reminder that awareness, compassion, and timely intervention are powerful tools in easing this burden.
By breaking stigma, encouraging early diagnosis, and strengthening support networks, societies can not only improve the quality of life for those living with Alzheimer’s but also bring hope to millions of families navigating its challenges.
NHL Star Rasmus Dahlin's Fiancée Received A Heart Transplant

Credits: Instagram and Canva
NHL and Buffalo Sabres star Rasmus Dahlin revealed in an open letter to hockey fans that his fiancée Carolina Matovac received a heart transplant. He noted that while the couple went to France on a vacation early this summer, she experienced a "major heart failure".
In the open letter, the Sabres captain detailed the traumatic experience. The team shared the letter on Friday. As per Dahlin, Matovac had been feeling ill for several days, which led to her experiencing "major heart failure".
He wrote: "Fortunately, she received CPR on multiple occasions, and up to a couple of hours at a time to keep her alive, which ultimately saved her life. Without her receiving lifesaving CPR, the result would have been unimaginable. It is hard to even think about the worst-case scenario."
As a result, Matovac also remained on life support for weeks before she received the transplant in France. He also wrote: "Without the incredible commitment, expertise, care, and sensitivity of all the people who treated Carolina, we would not be in the position that we are in today, with Carolina recovering well and on the path to a full recovery."
In his letter, he also thanked Matovac's medical team, the NHL and NHLPA, and the Pegula family for their support. He said that through his letter he hopes to remind others to "appreciate our experiences, the people closest to us, and the importance of fully living each day."
Update On Matovac's Health Now
He wrote that Carolina is still "working through her rehab to return to be with me in Buffalo, she has demonstrated an incredible determination, spirit, positivity, and resilience that I am in awe of." He called this the most challenging chapter of their lives, however, also noted that both have also learned a lot from this. He wrote: "We will continue to grow from these experiences and are so grateful for all the love and support we have received. We are truly blessed in so many ways and fully realize how fortunate we are."
What Is A Heart Failure?
Doctors reveal that unlike an actual heart attack, heart failure can happen gradually. This is also the reason why many people mistake the symptoms to be something else, like indigestion or being out of shape. Robert Greenfield, MD, cardiologist, and medical director of non-invasive cardiology and cardiac rehabilitation at MemorialCare Heart & Vascular Institute at Orange Coast Medical Center in Fountain Valley, tells Prevention, that "the longer you go without seeing these as signs of heart trouble, the more damage you may have over time.”
What actually happens in a heart failure is that your heart becomes unable to pump enough blood to meet the body's need. In the US, it happens to approximately 6.5 million adults in a year, and is the leading cause of hospital admissions and readmissions among older adults, accounting for an estimated 1 million hospitalizations annually, notes Naddi Marah, MD., FACC, FSCAI, interventional and structural cardiologist with Memorial Hermann Health System.
What Happens In A Heart Transplant?
A heart transplant replaces a falling heart with a healthy donor heart through complex open-chest surgery, lasting several hours. The recipient is placed under general anesthesia, connected to a heart-lung bypass machine, and their diseased heart is removed, while the donor heart is carefully sewn in the place, and blood vessels are attached so the heart begins to beat. Afterward, the patient is connected to mechanical ventilation, drainage tubes are inserted, and they receive immunosuppressant drugs to prevent rejection of the new organ.
© 2024 Bennett, Coleman & Company Limited

