- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Woman Left Screaming In Pain After Sex Toy 'Pulled Through Body' During MRI Scan
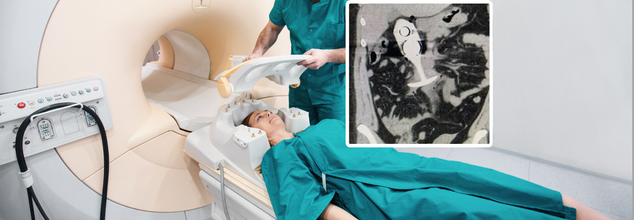
MRI scans are strong diagnostics with high-definition images of what lies inside a body. Strong magnetic fields require precaution, as brought out by an instance where a young woman suffered very serious injuries due to an oversight in a metallic core within a silicone sex toy that she happened to have before the MRI scan. This makes a stark reminder about the potentially deadly consequences of missing metal objects when such procedures are being performed. In April 2023, a 23-year-old woman went into an MRI with a silicone plug containing a metal core that was not known.
She thought that the item is made entirely out of silicone according to the advertising. However, the strong magnetic field of the MRI machine interacted with the hidden metal, dragging the object through her body and causing excruciating pain. According to reports from the U.S. Food and Drug Administration (FDA), the scene was harrowing, with the woman screaming in agony and requiring immediate hospitalization. Despite pre-scan screenings, which are routine prior to a scan, the patient did not inform the facility that the object existed because he presumed it was purely non-metallic. This caused serious injuries that led to the patient's law suit against the manufacturer for deceitful misrepresentations of material content.
MRI machines employ magnets between 0.5 to 3 Tesla (T). This is thousands of times stronger than the Earth's magnetic field. The tremendous force causes ferromagnetic materials, like iron and nickel, to be magnetized quickly and become strongly attracted toward the magnet. Objects as small as hairpins or paper clips will accelerate at 40 miles per hour inside the magnetic field.
The force can lead to catastrophic injuries in items lodged within the body, such as metallic implants or foreign objects. Metallic cores within devices, like pacemakers or intrauterine devices, must be disclosed to radiologists to prevent such complications.
How Metal Objects Interact with MRI Fields?
On these claims, Dr. Adam Taylor, a specialist in human anatomy, weighed his words in a international health website and added that the distance away and mass of this object would increase its velocity towards that of sound, "The acceleration would be phenomenal, but with a metallic core, it can't go anywhere near supersonic speeds. As for the size, the magnetic acceleration to the internal soft tissues would ensure that there could be severe intracranial trauma."
The injuries inflicted in this case likely involved damage to major blood vessels, nerves, or organs, highlighting the devastating impact of even minor oversight during an MRI scan.
This is not an isolated case. There are documented cases of metallic objects causing serious damage during MRI scans with a 65-year-old man with schizophrenia swallowed metal objects, including sockets and a hinge pin. The powerful magnetic field during an MRI scan caused the objects to rupture his stomach, resulting in serious injuries.
A toddler who ingested 11 small magnets perforated his bowel while undergoing a scan, making his case unique. In another deadly but extremely rare incident, there have been people who hide a firearm on themselves during MRI procedures. Magnetic attraction can trigger a discharge in a weapon and has led to some fatal injuries.
These cases emphasize the very strong need for adequate screening and patient education prior to an MRI.
Preventing MRI-Related Incidents
Medical professionals have been trained to avoid risks. This is by properly screening a patient for metallic objects. In general, most pre-scan protocols include:
- Patients are interrogated about implants, recent surgery or exposures at work related to metals.
- Radiologists sometimes use handheld metal detectors to search for hidden items.
- People who work with metal, like welders or machinists, will need additional testing to detect microscopic metal fragments within soft tissues or eyes.
The case emphasizes the importance of product labeling by manufacturers, especially those products that are likely to unintentionally cause harm to health. The patient's assumption that her device was 100% silicone points to a larger problem in consumer markets with misinformation.
It also reminds the patients to report any possible dangers to the medical professionals, no matter how the objects look non-metallic. In sensitive cases, patients can request private discussions with healthcare providers to ensure safety without discomfort.
In the end, it is a joint effort from manufacturers, healthcare professionals, and patients that can prevent such tragedies. Manufacturers must ensure truthful marketing, while healthcare providers should educate patients about the dangers of metal objects in MRI settings. For patients, understanding the risks and actively participating in pre-scan disclosures can be lifesaving.
This young woman's experience is a sobering example of the unforeseen dangers posed by MRI machines when precautions are overlooked. It serves as a wake-up call to address gaps in patient awareness, medical protocols, and product transparency. By learning from this incident, the medical community and the public can work together to ensure MRI scans remain a safe and effective diagnostic tool.
Nipah Virus Outbreak In India: How It Affects The Immune System

Credits: Canva and iStock
Nipah virus outbreak in India has triggered screenings across Asian airports. However, the health authorities of the Kolkata hospital where two nurses were admitted confirmed that one of the two nurses has been discharged from hospital. While this may be a good sign, there is still need to be cautious of the virus.
Nipah Virus Outbreak In India: How Does It Work?
Experts and doctors are still telling people to be cautious of what they eat, as the fatality rates are from 40 per cent to as high as 75 per cent. It is a zoonotic infections that infects vital organs like brain and lungs. However, the virus is also able to manipulate body's immune system.
Nipah Virus In India: How Does It Affect Immune System?
Nipah virus is lethal because it can outpace, suppress and misdirect immune responses. Speaking to NDTV, Dr Dip Narayan Mukherjee, Consultant, Microbiology and Infectious Diseases at CK Birla Hospitals said, Nipah virus leaves the body unable to clear the infection in time. "Understanding this immune disruption is critical to explaining why Nipah causes severe encephalitis, multi-organ failure and high mortality, and why early detection and containment remain the most effective tools against it."
This immune disruption begins early in the infection. The body’s first line of defense against viruses is the innate immune system, which relies heavily on interferons. These signalling proteins alert neighboring cells to the threat and trigger antiviral mechanisms that slow viral replication.
“One of the earliest ways Nipah evades immunity is by interfering with the innate immune response,” Dr Mukherjee says. “The virus suppresses interferon activity, allowing it to multiply rapidly before the immune system can respond adequately.”
Research published by the World Health Organization and other virology institutes shows that specific Nipah virus proteins block interferon signalling pathways. This gives the virus a crucial head start, enabling widespread infection before the immune system is fully activated.
Read: Australia Is Monitoring Nipah Virus Outbreak In India
Nipah Virus Outbreak In India: Inflammation And Low Immunity
As the infection progresses, Nipah targets the cells lining blood vessels, a feature that sets it apart from many other respiratory viruses. Damage to these vessels allows the virus to spread to multiple organs, including the brain, while also triggering widespread inflammation.
Instead of a controlled antiviral response, the body releases large amounts of inflammatory molecules. This excessive inflammation leads to tissue injury, swelling and organ dysfunction, contributing to respiratory failure, neurological symptoms and circulatory collapse in severe cases.
Another hallmark of Nipah infection is immune exhaustion. Although the virus does not directly infect most immune cells, the intense inflammatory environment causes them to become overactivated and eventually dysfunctional. Once these defense cells lose their ability to control viral replication, the infection accelerates, and supportive care becomes less effective in later stages.
When Nipah crosses into the brain, immune control becomes even more limited. The brain’s immune responses are naturally restrained to prevent damage, allowing the virus to persist. At the same time, inflammation causes swelling, seizures and encephalitis. Neurological complications remain the leading cause of death in Nipah outbreaks.
Nipah Virus Outbreak In India: Why Antibodies Against The Infection Arrive Late
The adaptive immune response, which includes antibody production and virus-specific T-cells, also struggles to keep pace with the rapid spread of Nipah. By the time neutralizing antibodies are produced, significant organ damage may have already occurred, particularly in the brain. This delayed response explains why severe encephalitis is common even in people without underlying health conditions.
AI Detects More Breast Cancer Cases in Landmark Swedish Study
Credit: Canva
Using artificial intelligence (AI) in breast cancer screening can reduce the number of cancers diagnosed in late stages by 12 percent, according to a major new study from Sweden.
The study found that fewer women in the AI group were diagnosed with breast cancer in the years after screening. There were 1.55 cancers per 1,000 women in the AI-supported group, compared with 1.76 per 1,000 in the standard screening group.
According to lead author Dr Kristina Lang of Lund University in Sweden, this indicates better early identification of clinically relevant cancers. She said of the results: “Our findings show that AI-supported screening improves the early detection of breast cancers that are more likely to become aggressive or advanced.
“This results in fewer serious cancers being diagnosed in the interval between screenings.”
She added that wider adoption of AI-supported mammography could ease workforce pressures on radiologists while improving early detection, including of aggressive cancer subtypes.
What Did The Study Find?
The study, published in The Lancet, involved around 100,000 women who took part in routine mammography screening between April 2021 and December 2022, making it the first large randomised trial to assess how AI performs in real-world breast cancer screening.
Women were randomly divided into two groups. One group received standard screening, where mammograms were read by two radiologists and the other group had AI-supported screening, where an AI system assessed the scans first.
Low-risk cases were read by one radiologist, while higher-risk cases were checked by two, with the AI also flagging suspicious areas.
The results showed that 81 percent of cancers in the AI-supported group were detected during screening, compared with 74 percent in the standard screening group—a nine percent increase. Importantly, false-positive rates remained similar, at 1.5 percent in the AI group and 1.4 percent in the control group.
Despite positive results, Dr Lang cautioned that introducing AI into healthcare must be done carefully, using validated tools and continuous monitoring to understand how performance may vary across regions and over time.
READ MORE: This 2 Hour Activity Can Reduce Your Breast Cancer Risk, Study Shows
Breast Cancer: A Rising Crisis
About 1.9 lakh Indian women are diagnosed with breast cancer annually, meaning that a new case is diagnosed every four minutes. On average, a woman in India dies of breast cancer every eight minutes, highlighting how urgently the country needs stronger awareness, early diagnosis and sustained care.
One factor that sets India apart is the age at which women are affected. Almost half of all breast cancer patients in the country are younger than 45. This is a much higher proportion than seen in many Western nations, where the disease is usually detected later in life.
Moreover, sedentary habits, excessive consumption of processed foods as well as alcohol and smoking promotes obesity and hormonal changes which pave the way for breast cancer development.
Union Budget 2026 Agriculture Push Will Improve Public Health, Experts Say

Credit: Canva
During today's Union Budget, Finance Minister Nirmala Sitharaman announced multiple initiatives and a staggering ₹1,62,671 crore to increase agricultural production in the country
Sitharaman, who was presenting her ninth consecutive budget, said the government will support the growth of high-value crops such as coconut, sandalwood, cocoa and cashew in coastal regions, agarwood in the North East and nuts,s including walnuts, almonds and pine nuts.
Dedicated programmes will focus on rejuvenating old orchards, expanding high-density cultivation and promoting value addition by engaging rural youth, while new coconut promotion schemes aimed at boosting productivity will be launched.
She noted: "India is the world’s largest producer of coconuts. About 30 million people, including nearly 10 million farmers, depend on coconuts for their livelihood. To further enhance competitiveness in coconut production, I propose a Coconut Promotion Scheme to increase production and enhance productivity through various interventions 16 including replacing old and non-productive trees with new saplings/plants/varieties in major coconut growing states.”
And health experts across the country say that an increase in consumption of these food items can significantly help improve your health.
Dr Sunil Kutty, Director and Consultant Brain and Spine Surgeon, NewEra Hospitals, Navi Mumbai, exclusively told Healthandme: "The Union Budget emphasises improving national nutrition and food security, recognising that better diets are foundational to health and well‑being.
"By strengthening food systems, supporting high‑value crops and enhancing access to nutrient‑rich foods, the government aims to reduce malnutrition, micronutrient deficiencies and diet‑linked diseases like Type-2 diabetes, obesity, and cardiovascular diseases. Integrating nutrition‑focused initiatives with healthcare can lower the overall burden of non‑communicable conditions and improve immunity, especially among children, women and vulnerable groups.
"These measures also support rural livelihoods, increasing access to affordable, healthy food across socio‑economic groups, contributing to long‑term health outcomes and reduced healthcare costs."
Coconut: Prevents Cell Damage And Packed With Essential Minerals
When eaten in moderation, coconuts offer healthy fats (MCTs), fibre, manganese, copper, iron, potassium as well as ample of antioxidants. Potassium helps balance sodium levels in the body and potentially lowers blood pressure, while the fruit's high fibre content supports bowel health and prevents constipation.
Coconuts are also especially high in manganese, which is essential for bone health and the metabolism of carbohydrates, proteins, and cholesterol. Along with this, they’re also rich in copper and iron, which help form red blood cells, as well as selenium, an important antioxidant that protects your cells and reduces cell damage which can prevent future cancer development.
A 2020 case study found that supplementing with coconut oil helped lower blood sugar levels in a person with diabetes, a condition characterized by unstable blood sugar levels. The researchers suggest that these effects may be due to the coconut’s anti-inflammatory properties and antioxidant content.
A 2024 animal study also found that consuming coconut water after eating could help manage blood sugar levels. This could be due to bioactive compounds like ellagic acid, butin, and quercetin, among others.
Sandalwood: Skin Benefits and Anxiety Reduction
Sandalwood offers benefits for skin health (acne, ageing, brightening), mental well-being, aid sleep, and possesses anti-inflammatory, antiseptic as well as antibacterial properties, making it useful for skin irritations, respiratory issues, and even potentially lowering blood pressure.
A 2017 Sigma study suggests that lavender, sandalwood, and orange-peppermint aromatherapy helped reduce self-reported feelings in anxiety of 87 women undergoing a breast biopsy.
In a 2016 pilot study published in NPC of 32 people in Vienna, Austria, participants inhaled lavender and sandalwood oil. The study found that the participants’ blood pressure levels were lower and that the cortisol levels in their saliva were lower after the aromatherapy.
Cocoa: Boosts Heart Health
While cocoa is most famous for its role in chocolate production, it is also packed with polyphenols and reduces high blood pressure by improving nitric oxide levels.
Researchers have previously linked polyphenols to numerous health benefits, including reduced inflammation, lower blood pressure and better heart health. It also contains flavanols, which have potent antioxidant and anti-inflammatory effects.
The flavanols in cocoa also improve nitric oxide levels in the blood, which can enhance the function of the blood vessels and reduce blood pressure.
Nuts: Improve Overall Health
Doctors say nuts offer heart-healthy fats, protein, fibre, vitamins, and minerals, which can lower heart disease and diabetes risk, improve cholesterol, reduce inflammation, and support weight management when eaten in moderation as part of a balanced diet.Over time, this can improve artery health, lower bad LDL cholesterol, and reduced risk of blood clots, with varieties like almonds, walnuts, and pistachios providing unique nutrients like omega-3s, antioxidants and selenium.
Lastly, Pattabhi Rama Rao, Managing Director, Gourmet Popcornica also noted to Healthandme: "These measures, combined with stronger market linkages and local enterprise development, can make wholesome foods more affordable and accessible, directly supporting better nutrition and overall public health outcomes across the country. Not just this, farmers being able to access equitable income will enable them to access high-quality, nutritious food.
"By prioritising high-value and nutritious crops like coconut, cashew, cocoa, and walnuts, the government is not just enhancing farmers’ incomes but also ensuring that healthier, nutrient-rich foods are more widely available to the public.
"Diversifying production through fisheries, livestock, and region-specific crops, like maize in Andhra Pradesh and Chhattisgarh, will improve access to proteins, healthy fats, and essential micronutrients, which are vital for balanced diets.
© 2024 Bennett, Coleman & Company Limited

