- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
Skin Implant That Glows Green May Help Detect Illness In Advance, Scientists Reveal
Credits: AI Generated
Japanese researchers have developed a new form of wearable health technology that could reshape how illness is detected. The innovation takes the form of a “living skin” implant that emits a green glow when the body begins to show early signs of disease. Designed to sit within the skin, the implant offers a visible warning when internal health markers shift, potentially alerting users before symptoms become obvious.
How The Glowing Implant Works
The implant works by monitoring internal biomarkers, which are proteins linked to inflammation, stress, and disease processes. When these biomarkers move outside their normal range, the implant responds by glowing green. Rather than depending on a smartwatch or fitness tracker, this approach would allow people to see changes in their health directly on their skin, offering continuous feedback throughout the day and night.
The project was developed by scientists from Tokyo City University and the University of Tokyo, with technical support from engineers at RIKEN and Canon Medical Systems. The team has already tested the concept in mice, where the implant functioned as a living sensor display embedded in the skin.
Why Researchers Sought a New Approach
The idea behind the technology grew out of the limitations of current health monitoring tools. According to Professor Hiroyuki Fujita, who was involved in the study, many existing methods are either invasive or provide only brief snapshots of a person’s health. “Conventional approaches are often invasive or provide only snapshots in time,” he explained. “Our goal was to explore a biologically integrated system that enables continuous sensing and intuitive interpretation, even at home.”The research was also featured in the Daily Star as part of The Weird Science Drop newsletter, which highlighted a range of unusual scientific developments alongside this project.
No Batteries, No Replacements
One of the most striking aspects of the implant is what it does not need. The sensor is made from living epidermal stem cells, allowing it to survive through the skin’s natural regeneration process. Because it is maintained by the body itself, the implant does not require batteries or external power sources, as per Express UK.
Professor Fujita noted that this makes the technology fundamentally different from traditional devices. “Unlike conventional devices that require power sources or periodic replacement, this system is biologically maintained by the body itself,” he said. In laboratory experiments, the sensor remained functional for more than 200 days, with the engineered stem cells continuously renewing the skin while keeping the implant active.
Potential Uses Beyond Human Health
As per Express UK, while the technology is still being tested, the researchers believe its applications could extend well beyond human healthcare. They suggest it may be especially useful in animal research and veterinary medicine. Since animals cannot describe how they feel, a visible signal on the skin could help detect illness earlier and allow for faster intervention.
Although the implant is not yet ready for everyday use, it offers a glimpse into a future where health monitoring becomes part of the body itself, quietly working in the background and providing clear visual cues when something is not right.
Chocolate Products Recalled In US Over Possible Salmonella Contamination

Credits: Canva
Specialty chocolate bars are being removed from store shelves after concerns were raised about possible salmonella contamination, the US Food and Drug Administration said on January 12. Spring & Mulberry has announced a voluntary recall of its Mint Leaf Date-Sweetened Chocolate Bar, which has been sold online and at selected retail locations across the country since September, the FDA confirmed.
The issue was flagged during routine testing carried out by an independent laboratory. So far, no illnesses or negative health effects linked to the product have been reported.
As per The Independent, consumers who purchased the recalled chocolate are being advised to throw it away and seek a refund from Spring & Mulberry. The company has asked customers to contact them directly and provide a photograph of the chocolate bar showing the affected lot code.
Chocolate Bars Sold Across The US Recalled Over Salmonella Concerns
A well-known chocolate brand has issued a recall after possible salmonella contamination was detected. Spring & Mulberry, a Raleigh, North Carolina-based company, voluntarily recalled its Mint Leaf Date-Sweetened Chocolate Bar following routine third-party testing that found traces of salmonella, according to an FDA notice released Monday.
The recalled bars have been sold online and through selected retail partners since September 15, 2025. Consumers can identify the affected product by its teal-colored packaging and the lot code 025255 printed on the back of the box and the inner wrapper.
What Is Salmonella?
Salmonella is a type of bacteria that can cause serious and, in some cases, life-threatening infections, particularly in young children, older adults, and people with weakened immune systems, according to the FDA. Symptoms of salmonella infection may include:- Bloody diarrhea or diarrhea that lasts more than three days without improvement
- Diarrhea accompanied by a fever above 102 degrees
- Severe vomiting that makes it difficult to keep fluids down
- Signs of dehydration such as dry mouth, reduced urination, and dizziness when standing
- Abdominal cramps
Symptoms usually appear between six hours and six days after exposure. While most people recover within four to seven days, those at higher risk, including children under 5 and adults over 65, may develop more severe illness that requires medical care or hospitalization.
Affected Lot Code For Recalled Chocolates
The recalled Spring & Mulberry chocolate carries the lot code #025255. This code applies specifically to the Mint Leaf Date-Sweetened Chocolate Bar that has been pulled from the market due to the potential risk of salmonella contamination identified during routine lab testing.
Shoppers are urged to check their packaging carefully for the listed lot code. Anyone who has a bar with the affected code should not eat it. Instead, they should discard the product and contact Spring & Mulberry to request a refund.
As per USA TODAY, although no illnesses have been reported so far, the recall has been issued as a precaution to safeguard public health, especially for vulnerable groups such as children, older adults, and people with compromised immune systems. The FDA has shared further guidance on recall steps and salmonella symptoms.
The chocolate recall follows another FDA action just months earlier involving Doughy Chocolate Chip Cookie Dough produced by Hudson River Foods in November, also due to possible salmonella contamination. That recall was later upgraded to a Class I recall on December 5 after officials determined that consuming the product could result in serious health effects or death.
A total of 113 units of the edible cookie dough were included in that recall. The affected products were sold in 12-ounce containers and carried a best-by date of July 4, 2026.
Wegovy 7.2 mg: Higher-Dose Weight-Loss Jab Cleared For Launch In UK
Credits: AI Generated
The UK’s drug regulator has cleared a higher-strength dose of the weight-loss injection Wegovy, as demand for the treatment is expected to rise sharply. The newly approved 7.2 mg dose is three times stronger than the current weekly dose of 2.4 mg. The Medicines and Healthcare products Regulatory Agency, or MHRA, said the stronger dose can lead to weight loss of more than 20 per cent. Trial data reviewed by the regulator showed that around one in three adults living with obesity who took the higher weekly dose lost more than 25 per cent of their body weight after 72 weeks.
Weight Loss Jab To Launch Stronger Dosage In UK
The MHRA’s latest approval for Wegovy is based on findings from a clinical study known as STEP UP, which looked at how patients responded to the treatment. Results showed that people taking the 7.2 mg dose lost an average of 20.7 per cent of their body weight, compared with a 2.4 per cent reduction among those given a placebo. The trial also noted side effects, with mild to moderate digestive problems reported most often.The approval comes alongside research from the University of Oxford suggesting that people using drugs such as semaglutide, sold as Wegovy, and tirzepatide, marketed as Mounjaro, tend to lose weight while on treatment but regain it within about 20 months after stopping the injections. According to The Independent, people who lose weight through diet and exercise tend to maintain the loss for longer, close to four years on average, although some regain still occurs over time.
Researchers cautioned that people using weight-loss injections need long-term support, as their analysis showed weight returns more quickly after stopping the drugs than it does for those following traditional diet plans. The study also found that improvements in blood sugar, cholesterol levels, and blood pressure fade once the medication is discontinued, leaving many patients back where they started. The Oxford-led research, published in the British Medical Journal, reviewed 37 studies involving more than 9,000 participants.
What Is Wegovy?
Wegovy is a widely used weight-loss injection containing the active ingredient semaglutide. Studies show that people on the standard maintenance dose of 2.4 mg lose an average of 17 per cent of their body weight over 68 weeks. A higher 7.2 mg dose has now been approved for use in the UK, with clinical trials indicating it can deliver weight loss of up to 21 per cent.
What is Wegovy 7.2mg?
Wegovy 7.2 mg is a new maintenance dose of the semaglutide-based weight-loss injection. Until now, the highest approved weekly dose was 2.4 mg. The stronger 7.2 mg option is being introduced by the drug’s manufacturer, Novo Nordisk, following its submission to regulators for approval.
This new dose uses the same active ingredient, semaglutide, and works in the same way by helping overweight or obese adults lose weight. It does this by copying the action of the hormone glucagon-like peptide-1, or GLP-1, which helps regulate appetite and food intake.
Further studies examining the safety and effectiveness of the higher dose are already in progress. The latest findings from the STEP UP phase 3b trial were published in November 2025.
How Effective Is The Wegovy 7.2 Dose?
The STEP UP phase 3b trial assessed how safe and effective the 7.2 mg dose of Wegovy is. The study included 1,407 participants who were randomly assigned to receive either 7.2 mg or 2.4 mg of Wegovy, or a placebo, over an 11-month period.
The average weight loss recorded was:
- 20.7 per cent in the 7.2 mg group
- 17.5 per cent in the 2.4 mg group
- 2.4 per cent among those given a placebo
The approval follows recent findings showing that about 1.6 million people used Wegovy or Mounjaro last year. Another 3.3 million people said they would consider using weight-loss medications in the coming year.
According to The Independent, research by University College London based on a survey of 5,260 people found that 2.9 per cent of respondents were using GLP-1 drugs for weight loss. Of those, 15 per cent were taking medication that is not licensed for that purpose. Researchers warned that using drugs off label, meaning for conditions they are not approved to treat, such as using diabetes drugs for weight loss, can carry safety risks if taken without proper medical supervision.
Measles Spreads Rapidly In South Carolina, More Than 400 Cases Confirmed
Credits: Canva
The Upstate South Carolina measles outbreak grew to 434 confirmed cases by Tuesday, officials reported. Health authorities said the outbreak has expanded quickly, with 124 new cases recorded in just a few days between January 9 and January 13, 2026.
According to the South Carolina Department of Public Health, around 409 people were placed under quarantine and 17 were kept in isolation as of Tuesday. Officials also confirmed that one individual, unaware they were contagious at the time, visited the South Carolina State Museum in Columbia on January 2 between 1 pm and 5 pm. Anyone who was at the museum during that window may have been exposed and has been advised to watch closely for symptoms.
What Is Measles?
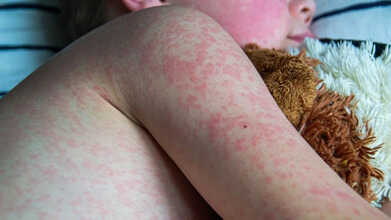
Measles, also known as rubeola, is a highly contagious viral illness that typically causes fever, cough, runny nose, red eyes, and a widespread red rash. It spreads through airborne droplets released when an infected person coughs or sneezes. The virus is extremely infectious and can remain in the air or on surfaces for up to two hours.
While some cases are mild, measles can lead to serious complications such as pneumonia, swelling of the brain, and even death, especially in young children. A safe and effective vaccine has prevented millions of deaths worldwide, but outbreaks continue to occur in areas with low vaccination coverage, according to the Mayo Clinic.
Measles Cases Spreading In The US
South Carolina health officials said yesterday that the state’s measles count has reached 434 after confirming 124 new cases. At present, 409 residents are in quarantine and 17 are in isolation, with some quarantine periods expected to last until February 6.
Mobile vaccination units are operating this week, and officials are strongly encouraging residents to get vaccinated. “Getting vaccinated now can help people avoid long periods of quarantine at home after exposure to the measles virus. Vaccination within 72 hours of exposure can prevent measles infection,” the state’s department of public health said in its latest update.
Measles Symptoms
Measles can result in hospitalization and, in severe cases, death. Common symptoms include:
- High fever, which can rise above 104 degrees
- Cough
- Runny nose
- Red, watery eyes
- Tiny white spots inside the cheeks, gums, and on the roof of the mouth known as Koplik spots, appearing two to three days after symptoms begin
- A red, raised, blotchy rash that usually starts on the face and spreads to the trunk, arms, and legs within three to five days after symptoms start
Measles Vaccine Prevention
According to the US Centers for Disease Control and Prevention, the measles, mumps, and rubella or MMR vaccine is 97 percent effective against measles and 86 percent effective against mumps when both recommended doses are received.
The MMR vaccine is part of the routine childhood immunization schedule. The first dose is typically given between 12 and 15 months of age, followed by a second dose between ages 4 and 6. Children who are traveling internationally may receive the vaccine earlier.
As reported by CIDRAP, South Carolina allows religious exemptions from vaccination through a notarized form, without requiring a doctor’s approval. One of the schools linked to the early phase of the outbreak, Global Academy of South Carolina, reported a vaccination rate of just 17 percent during the 2024 to 25 school year.
Of the 434 measles cases tracked over the past six months, 378 patients were unvaccinated and 47 had an unknown vaccination status. Only six people were fully vaccinated, while three had received partial vaccination. About two thirds of all measles cases in South Carolina involve children and teens aged 5 to 17, accounting for 287 cases.
© 2024 Bennett, Coleman & Company Limited

