- Health Conditions A-Z
- Health & Wellness
- Nutrition
- Fitness
- Health News
- Ayurveda
- Videos
- Medicine A-Z
- Parenting
FDA Recalled The Common ADHD Medication Vyvanse
Credits: Canva
Common ADHD medication Vyvanse has been recalled by the Food and Drug Administration, US, the last week. As per FDA, the lots of a prescription ADHD medication failed dissolution specification. As a result Sun Pharmaceutical Industries of New Jersey recalled bottles of lisdexamfetamine dimesylate capsules, which is a generic version of Vyvanse that was sold across the country.
the recall was initiated on October 28,and was categorized under Class II risk level by the FDA on October 30. As per FDA, a Class II recall means that the recalled medication “may cause temporary or medically reversible adverse health consequences,” however, the risk of any serious health consequences is low.
Which Medications Are Recalled?
Here is how you can check the lot number to know whether the medication you have in your cabinet has been recalled:
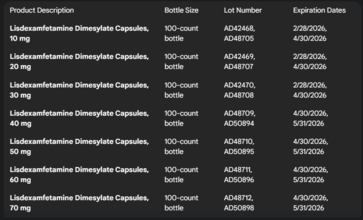
Sun Pharmaceutical Industries recalled the medication due to “failed dissolution specifications,” meaning the drug did not dissolve as expected in laboratory settings, which could make it less effective.
What Should You Do?
While there is no specific instruction for what the consumers should do as there is no official press release by the manufacturer, however, speak to your doctor before stopping your ADHD medications as an abrupt stop could lead to withdrawal symptoms.
What Is A Dissolution Test?
This test is conducted to determine the compliance of the drug with the dissolution requirements for dosage forms to be administrated orally.
Dissolution specifications are quality control limits for drug products that define the acceptable amount of drug substance that must dissolve in a specified time under laboratory conditions. The test also ensures batch-to-batch consistency and predicts how a drug will be released in the body, providing a surrogate measure of clinical performance.
The specifications are based on data from batches, which are used in clinical trials and are then guided by the drug's properties and the dosage form. They vary depending on the drug release types, which could be categorized under three kinds:
- Immediate Release (IR)
- Extended Release (ER)
- Delayed Release (DR)
What Does FDA Say About This Recall?
As per the FDA, Class I recalls are the most serious, this is where there is a reasonable probability that using or being exposed to the recalled drug could cause serious health consequences to the customer. This recall involves removing the drug from the market and are conducted at consumer level.
Class II recall however could cause temporary health consequences but the probability of health issue is remove. FDA notes that "these recalls are generally conducted at the retail level, and patients and consumers can continue using the medicine unless otherwise directed by the recalling company or FDA."
FDA says that for class II or class III recalls, consumers may "generally continue taking the medicine unless the recalling company provides other instructions".
'Don’t Do Drugs, Kids', Says Paris Jackson As Shows The Holes In Her Nose After Drug Use

Credits: Wikimedia Commons, IMDb, TikTok
Paris Jackson, the 27-year-old daughter of the late Michael Jackson opened up about drug earlier indulgence in drugs. In a TikTok video, she showed off her 'perforated septum'. She said, "I have a really loud whistle when I breathe through my nose. And that is because I have what is called a perforated septum," she explained, noting it’s "slightly different from a deviated septum."
She said this happened from drugs and said in her video "That is from what you think it's from. Don't do drugs, kids."
Paris Jackson shared with her fans about her six-year achievement of staying sober, however, she says that she still lives the consequences. However, she notes that she currently does not plan to undergo surgery to fix her nose, because "you have to take pills when you have a surgery that gnarly."
Perforated Nose From Drug Use
This is commonly called the Coke Nose. A 2013 study from the Iranian Journal of Otorhinolaryngology states that perforation of the nasal septum is an uncommon condition. When it occurs, its cause is most often idiopathic or traumatic. Nasal septum perforation may also be the presenting sign of drug addiction or a potentially life-threatening or serious systemic illness, even in an asymptomatic patient. This happens due to intranasal drug abuse, common with cocaine, heroin, and other opioids.
What Is A Coke Nose?
It is a colloquial term used for the nasal damage caused by prolonged cocaine use. Damage can range from mild to severe, leading to conditions such as a deviated septum, a perforated septum (hole in the nose from coke), or saddle nose deformity. People who inhale its vapors (known as freebasing) or snort cocaine through their nose put themselves at risk for the following conditions.
Chronic nasal insufflation (snorting) of cocaine often causes recurrent, painful sinus infections. This is particularly severe in users with damage like nasal perforations, or a palatal perforation (a hole between the mouth's roof and nasal cavity).
The persistent damage and irritation enable bacteria or fungus to colonize the sinuses. Such infections can be serious, potentially increasing tissue loss, causing deformities, and leading to life-threatening conditions.
Chronic cocaine use can cause septal perforations in the nose and may also lead to a hole (perforation) in the oral palate. This perforation connects the mouth and nasal cavity, causing food and drink to leak into the nasal area. Since the nasal cavity cannot properly process food remnants, these remnants often get stuck in the sinuses, significantly increasing the risk of sinus infections and related issues.
What Are The Common Symptoms Or Signs Of Coke Nose?
- Frequent nosebleeds
- Frequent runny nose
- Recurrent sinus infections
- Difficulty breathing
- Sleep apnea
- Noisy breathing
- Nose deformity
Can Coke Nose Be Treated?
Fortunately, many long-term effects of drug abuse are reversible or treatable. However, long-term resolution for conditions requiring surgical options, such as saddle nose correction or correction of septal or palatal perforations, depends entirely on the cessation of cocaine use.
Byheart Formula Recall Update: 2 More Infants Hospitalized, FDA Says

Credits: Canva
Health and Me had previously reported on the recall of ByHeart Baby Formula after 10 US states reported 13 cases of infant botulism after its use. Ever since the botulism outbreak, two more cases of infant botulism was reported, making the outbreak across 12 US states, as confirmed by the Food and Drug Administration (FDA), US's advisory.
As of now, a total of 15 cases of infant botulism has been reported, confirmed the Centers for Disease Control and Prevention (CDC), US.
FDA in its advisory said, "For 14 cases with illness onset information available, illnesses started on dates ranging from August 9 to November 10, 2025. All 15 infants were hospitalized."
From the information available, of the 14 infants, the age ranged from 16 days to just over 5 months, and half of them were females. The FDA confirmed that the infants who fell ill were all fed ByHeart Whole Nutrition powdered infant formula.
The multistate outbreak is now being investigated by FDA, CDC, and California Department of Public Health Infant Botulism Treatment and Prevention Program, and other state and local health authorities, the advisory stated. On Tuesday, ByHeart informed that the company is expanding its earlier recall in order to include all batches of the baby formula and the Anywhere Pack single-serve sticks nationwide.
"The safety and well‑being of every infant who uses our formula is, and always will be, our highest priority. This nationwide recall reflects our commitment to protecting babies and giving families clear, actionable information. Alongside this recall, we are conducting a comprehensive investigation to do our part to get the answers parents expect and deserve," said Mia Funt, the president and co-founder of the company.
What Is Infant Botulism?
Most common form of all botulism in babies, who are between 2 to 8 months old. It happens when the bacteria spores grow in a baby’s intestines and produce the toxin. Honey and contaminated soil can be sources of infant botulism. Adults can also get this type, though it’s rare.
What Are The Symptoms Of Botulism In Infants?
As per CDC, the symptoms include:
- Most infants with infant botulism will initially develop constipation, poor feeding, loss of head control, and difficulty swallowing
- If untreated, infants with infant botulism experience a progressive flaccid paralysis that can lead to breathing difficulties and required weeks of hospitalization.
States Hit By Infant Botulism Outbreak:
As per the FDA advisory, here is the list of states affected by infant botulism:
- Arizona
- California
- Illinois
- Kentucky
- Minnesota
- North Carolina
- New Jersey
- Oregon
- Pennsylvania
- Rhode Island
- Texas
- Washington
The company ByHeart Inc's two lots of Whole Nutrition Infant Formula has been recalled, the lots are:
- Lot: 206VABP/251261P2 ("Use by 01 Dec 2026")
- Lot: 206VABP/251131P2 ("Use by 01 Dec 2026")
What Parents Should Do?
As per the FDA advisory, parents and caregivers should stop using any ByHeart infant formula products immediately. Furthermore, the advisory notes:
- If your child consumed ByHeart formula and is experiencing symptoms (see below) seek immediate medical attention.
- If your child consumed ByHeart formula and is not currently showing symptoms, continue monitoring them and seek medical attention if symptoms develop.
- If you still have the formula in your home, you should:
•keep the container in a safe spot and be sure to label that product as DO NOT USE.
•If your child develops symptoms your state health department might want to collect your formula container for testing. If your child does not develop symptoms after 30 days, throw your containers out.
Canada Loses Measles Elimination Status Amid Massive Outbreak—Could US Face The Same Threat?
Credits: Canva
Canada has officially lost its measles elimination status, the Public Health Agency of Canada announced on Monday, following a large, ongoing outbreak of the virus. The Pan American Health Organization (PAHO), part of the World Health Organization, informed Canada that viral transmission has continued without interruption since October 2024.
Although the spread has slowed in recent weeks, the outbreak persists, mainly in communities with lower vaccination coverage.
What Is Measles?
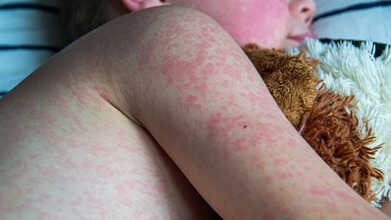
Measles is a highly contagious virus that can become life-threatening if not detected and treated early.
The infection spreads through the air when an infected person coughs or sneezes. It typically starts with symptoms similar to a common cold, such as a runny nose, high fever, and red, sore eyes.
A few days after the initial infection, tiny white spots may appear inside the cheeks and on the back of the lips. This is usually followed by a rash that begins on the face and behind the ears, eventually spreading across the body—the most recognizable sign of measles, according to NHS England.
Why Has Canada Lost Its Measles Status?
“PAHO’s Measles and Rubella Elimination Regional Monitoring and Re-Verification Commission reviewed the latest epidemiological and lab data, confirming ongoing transmission of the same measles strain in Canada for over a year,” officials said, as per CNN.
The Re-Verification Commission (RVC) is an independent group of experts that monitors measles outbreaks in the Americas and advises PAHO’s director, who makes the final determination regarding elimination status.
Canada had been considered measles-free since 1998, after successful vaccination campaigns following the approval of the measles vaccine in 1963. However, in recent years, vaccine hesitancy has grown, and immunization rates have dropped below the 95% coverage recommended for all childhood vaccines.
However, with Canada’s change in status, the Americas region as a whole has now officially lost its measles-free designation.
Can Canada Regain Measles-Free Status Again?
Canada can regain its elimination status once transmission of the outbreak strain is halted for at least 12 months. The Public Health Agency of Canada says it is working closely with PAHO and local public health authorities to control the outbreak
Can U.S. Lose Its Measles Elimination Status Too?
The United States has also experienced major measles outbreaks this year and is at risk of losing its elimination status. “We hope the U.S. can stop transmission before reaching that point,” said Dr. Daniel Salas, executive manager of PAHO’s Special Program for Comprehensive Immunization.
This year’s outbreak began in West Texas in January and has since spread to other states, with investigations ongoing into linked cases along the Arizona-Utah border.
Mexico has also reported a recent outbreak, mainly in Chihuahua, with additional cases in southern states.
Measles Vaccination Remains the Key
PAHO officials stress the importance of continued vaccination efforts. “With political commitment, regional cooperation, and sustained immunization, Canada and the Americas can once again halt transmission and reclaim measles-free status,” Barbosa said.
© 2024 Bennett, Coleman & Company Limited

